Thorium: Properties, Abundance, and Fissionability for UPSC - Science And Technology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search

Thorium: Properties, Abundance, and Fissionability for UPSC
Medium⏱️ 7 min read
science and technology
📖 Introduction
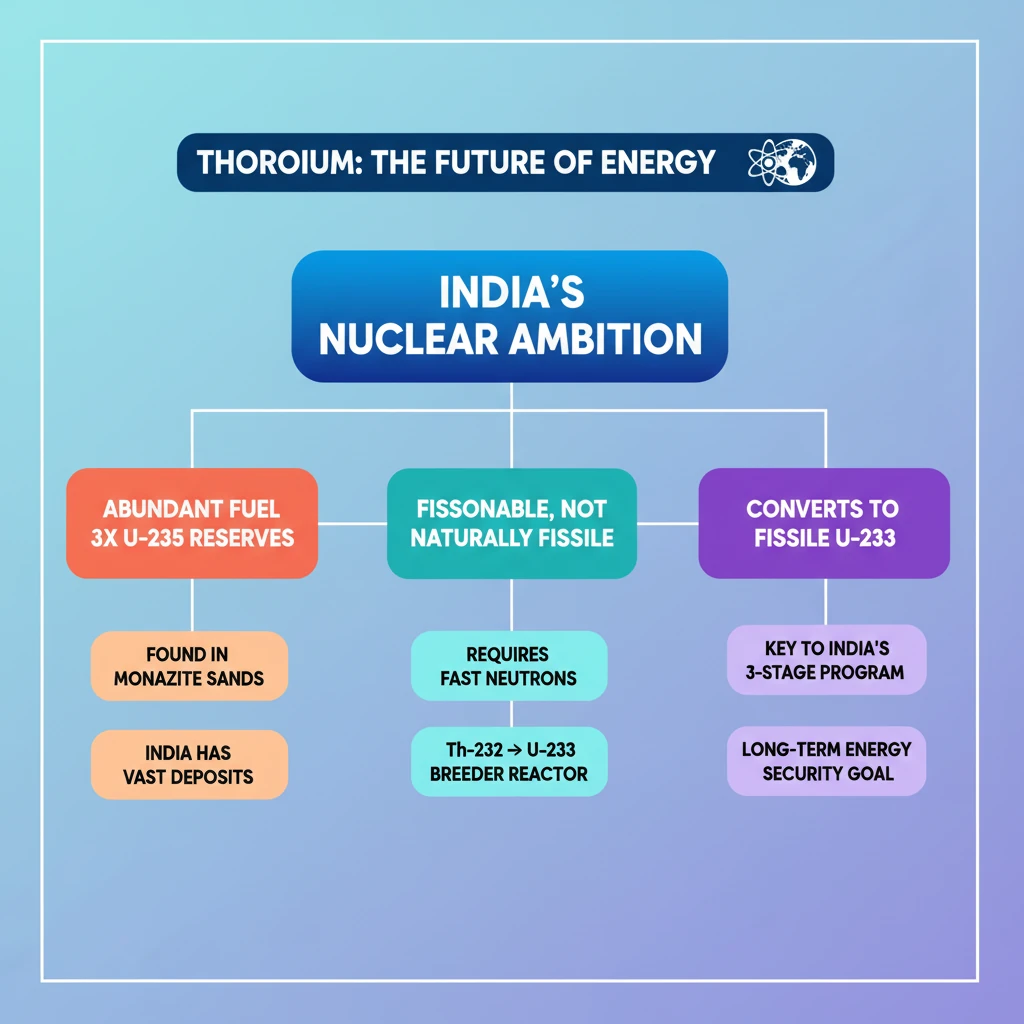
<h4>Introduction to Thorium</h4><p><strong>Thorium</strong> is a naturally occurring, slightly <strong>radioactive metal</strong>. It presents a silvery appearance and is found in various geological formations.</p><div class='info-box'><p><strong>Key Property:</strong> Thorium is a <strong>radioactive metal</strong>, meaning its atoms undergo spontaneous decay, emitting radiation.</p></div><h4>Occurrence and Distribution</h4><p>This metal is commonly present in <strong>igneous rocks</strong>, which are formed from the cooling of molten magma or lava. It is also found in significant concentrations within <strong>heavy mineral sands</strong>, often alongside other valuable minerals.</p><div class='info-box'><p><strong>Common Locations:</strong> Found in <strong>igneous rocks</strong> and <strong>heavy mineral sands</strong> globally.</p></div><h4>Global Abundance Compared to Uranium</h4><p><strong>Thorium</strong> is considerably more abundant in the <strong>Earth’s crust</strong> than <strong>uranium</strong>, a more commonly known nuclear fuel. Its average concentration is significantly higher.</p><div class='info-box'><p><strong>Abundance Data:</strong></p><ul><li><strong>Thorium:</strong> Approximately <strong>10.5 parts per million (ppm)</strong> in the Earth's crust.</li><li><strong>Uranium:</strong> Approximately <strong>3 ppm</strong> in the Earth's crust.</li></ul><p>This indicates that <strong>thorium</strong> is about three times more abundant than <strong>uranium</strong>.</p></div><h4>Fissionable but Not Fissile Nature</h4><p>The only naturally occurring isotope of <strong>thorium</strong> is <strong>Thorium-232</strong>. This isotope possesses a unique characteristic: it is <strong>fissionable</strong> but not <strong>fissile</strong>.</p><div class='key-point-box'><p><strong>Understanding the Distinction:</strong></p><ul><li><strong>Fissionable:</strong> An atomic nucleus that can undergo <strong>fission</strong> (splitting into smaller nuclei) when struck by a neutron.</li><li><strong>Fissile:</strong> An atomic nucleus that can sustain a <strong>nuclear chain reaction</strong> when struck by a <strong>thermal neutron</strong> (low-energy neutron).</li></ul></div><p>While <strong>Thorium-232</strong> can undergo <strong>fission</strong>, it cannot sustain a <strong>chain reaction</strong> on its own without external neutron assistance. It specifically requires <strong>high-energy neutrons</strong> to initiate and continue the fission process effectively.</p><div class='exam-tip-box'><p><strong>UPSC Insight:</strong> The distinction between <strong>fissionable</strong> and <strong>fissile</strong> is crucial for understanding nuclear energy and <strong>India's three-stage nuclear power program</strong>, which aims to utilize <strong>thorium</strong>.</p></div>
💡 Key Takeaways
- •Thorium is a silvery, slightly radioactive metal, three times more abundant than uranium.
- •It is commonly found in igneous rocks and heavy mineral sands, with India having significant reserves.
- •Thorium-232 is fissionable (can undergo fission) but not fissile (cannot sustain a chain reaction without external neutrons).
- •It requires high-energy neutrons to fission and can be converted to fissile Uranium-233.
- •Thorium is crucial for India's three-stage nuclear power program for long-term energy security.
🧠 Memory Techniques
98% Verified Content
📚 Reference Sources
•International Atomic Energy Agency (IAEA) publications on Thorium Fuel Cycle
•Department of Atomic Energy (DAE), Government of India reports
•NCERT Science textbooks (Class 10, 12 for basic atomic structure and radioactivity)