CAR-T Cell Therapy - Science And Technology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
CAR-T Cell Therapy
Medium⏱️ 8 min read
science and technology
📖 Introduction
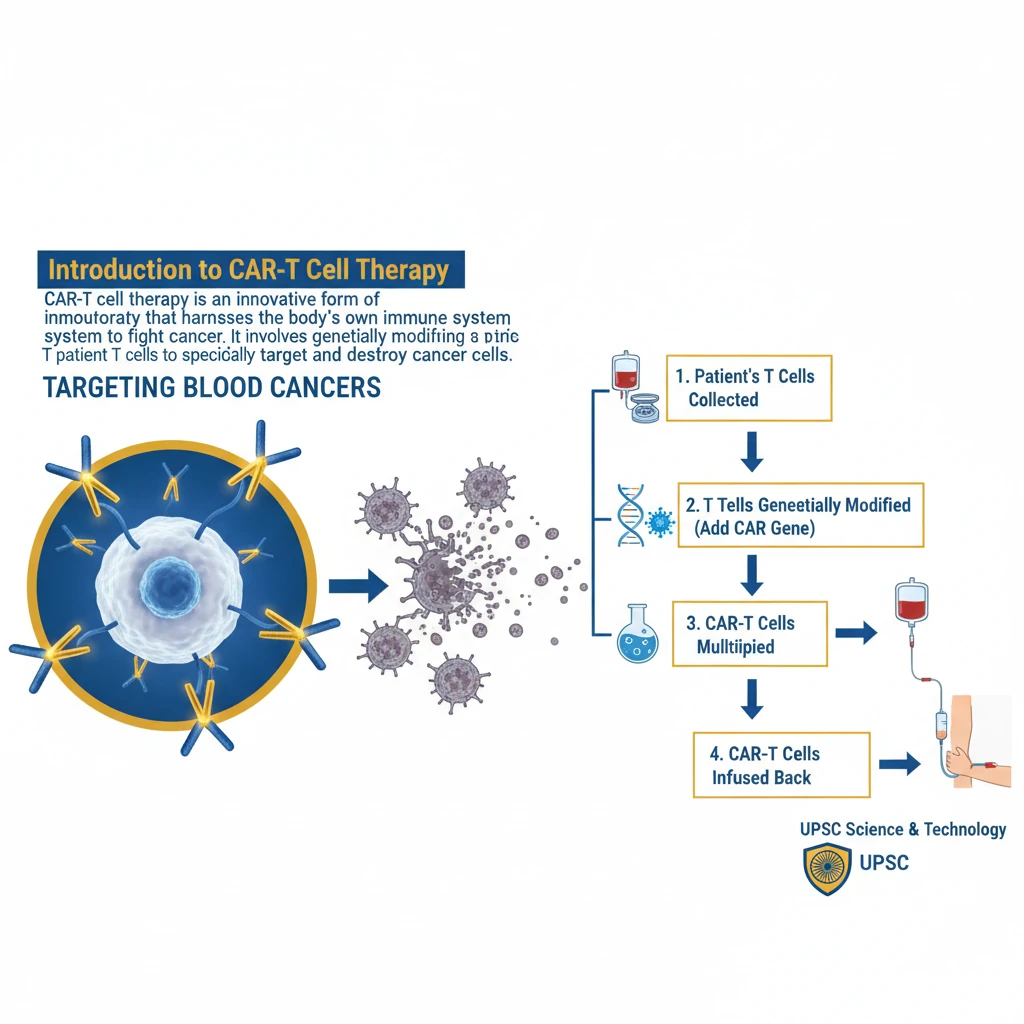
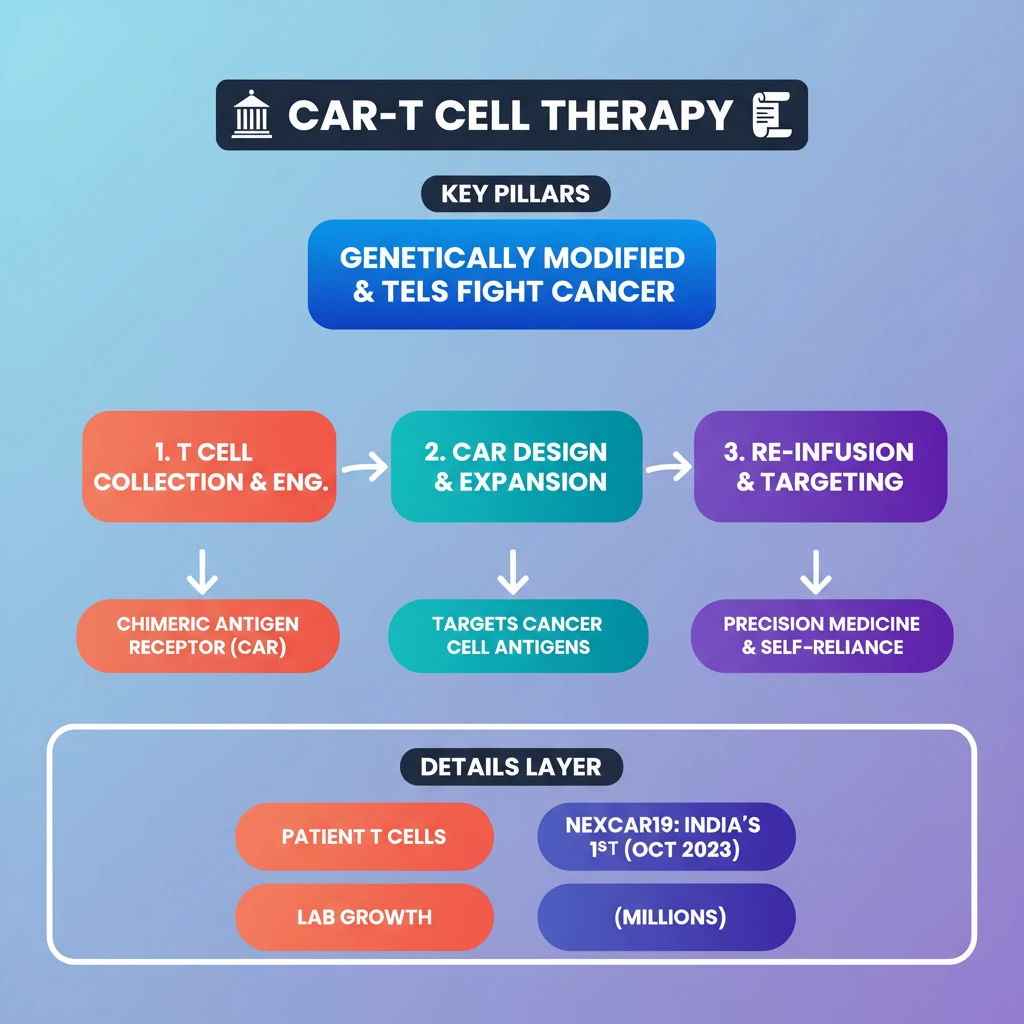
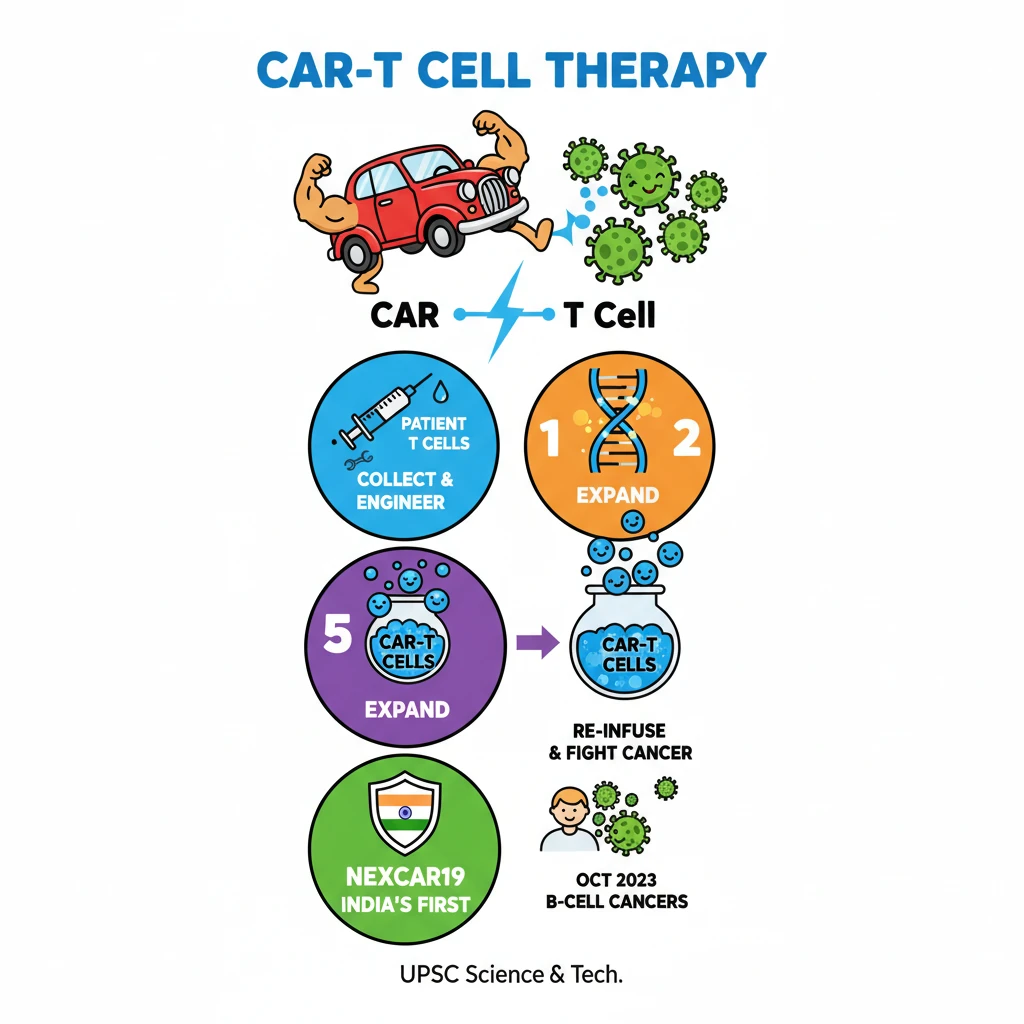
<h4>Introduction to CAR-T Cell Therapy</h4><p><strong>CAR-T cell therapy</strong> is an innovative form of <strong>immunotherapy</strong> that harnesses the body's own immune system to fight cancer. It involves genetically modifying a patient's <strong>T cells</strong> to specifically target and destroy cancer cells.</p><p>This advanced treatment represents a significant breakthrough, particularly in treating certain types of <strong>blood cancers</strong>, offering new hope for patients who have exhausted conventional therapies.</p><h4>Mechanism of CAR-T Cell Therapy</h4><p>The core principle of <strong>CAR-T cell therapy</strong> lies in equipping <strong>T cells</strong> with a specialized receptor. This receptor enables them to precisely identify and attack cancer cells.</p><div class='info-box'><p>A <strong>chimeric antigen receptor (CAR)</strong> is a synthetic protein engineered to recognize a specific <strong>antigen</strong> (marker) present on the surface of cancer cells. It combines the antigen-binding domain of an antibody with the signaling domains of a T cell receptor.</p></div><p>Once infused back into the patient, these engineered <strong>CAR-T cells</strong> act as 'living drugs,' actively seeking out and eliminating malignant cells throughout the body.</p><h4>Steps in CAR-T Cell Therapy</h4><p>The process of <strong>CAR-T cell therapy</strong> involves several critical stages, beginning with the collection of the patient's own immune cells.</p><ol><li><strong>Cell Collection:</strong> Healthcare providers collect blood from the patient to obtain their <strong>T cells</strong>. These T cells are then separated from the rest of the blood, which is returned to the patient.</li><li><strong>Genetic Engineering:</strong> In a laboratory setting, the isolated <strong>T cells</strong> are genetically modified. This modification involves introducing a gene that codes for the <strong>chimeric antigen receptor (CAR)</strong> onto their surface.</li><li><strong>CAR Design:</strong> The engineered <strong>CAR</strong> is specifically designed to recognize and bind to a particular <strong>antigen</strong> (marker) that is unique to the patient's cancer cells.</li><li><strong>Expansion:</strong> The genetically altered <strong>CAR-T cells</strong> are then multiplied in large numbers in the lab, creating millions of these specialized cancer-fighting cells.</li><li><strong>Pre-Infusion Chemotherapy:</strong> Before the <strong>CAR-T cells</strong> are infused, patients typically receive a course of <strong>chemotherapy</strong>. This helps to reduce existing immune cells, making space for the new CAR-T cells to expand and function effectively.</li><li><strong>Infusion:</strong> The expanded <strong>CAR-T cells</strong> are infused back into the patient’s bloodstream. Once inside, they can identify and attack cancer cells expressing the targeted antigen.</li></ol><h4>Development in India: NexCAR19</h4><p>India has made significant strides in indigenous development of <strong>CAR-T cell therapy</strong>, marking a crucial step towards self-reliance in advanced medical treatments.</p><div class='key-point-box'><p><strong>NexCAR19</strong> is an indigenously developed <strong>CAR-T cell therapy</strong> specifically designed for <strong>B-cell cancers</strong>. It is a collaborative effort by <strong>ImmunoACT</strong>, the <strong>Indian Institute of Technology Bombay (IIT-B)</strong>, and <strong>Tata Memorial Hospital</strong>.</p></div><p>The <strong>Central Drugs Standard Control Organisation (CDSCO)</strong> granted approval for the commercial use of <strong>NexCAR19</strong> to treat certain blood cancers in <strong>October 2023</strong>. This approval signifies a major milestone for advanced cancer care in India.</p><div class='exam-tip-box'><p>The development and approval of <strong>NexCAR19</strong> are highly relevant for UPSC, showcasing India's capabilities in <strong>biotechnology</strong> and <strong>healthcare innovation</strong>. It can be cited in questions related to 'Make in India' in the pharmaceutical sector and advancements in medical science.</p></div>
💡 Key Takeaways
- •CAR-T cell therapy uses genetically modified T cells to fight cancer.
- •It involves collecting patient T cells, engineering them with a Chimeric Antigen Receptor (CAR), expanding them, and re-infusing them.
- •CARs are designed to recognize specific antigens on cancer cells.
- •NexCAR19 is India's first indigenous CAR-T therapy, approved in October 2023 for B-cell cancers.
- •This therapy represents a major advancement in precision medicine and India's self-reliance in advanced healthcare.
🧠 Memory Techniques
98% Verified Content
📚 Reference Sources
•Official press releases and reports from ImmunoACT, IIT Bombay, and Tata Memorial Hospital regarding NexCAR19 approval.
•Central Drugs Standard Control Organisation (CDSCO) notifications.
•General scientific literature on CAR-T cell therapy (e.g., Cleveland Clinic, NCI).