What is Nitrogen Pollution? - Science And Technology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
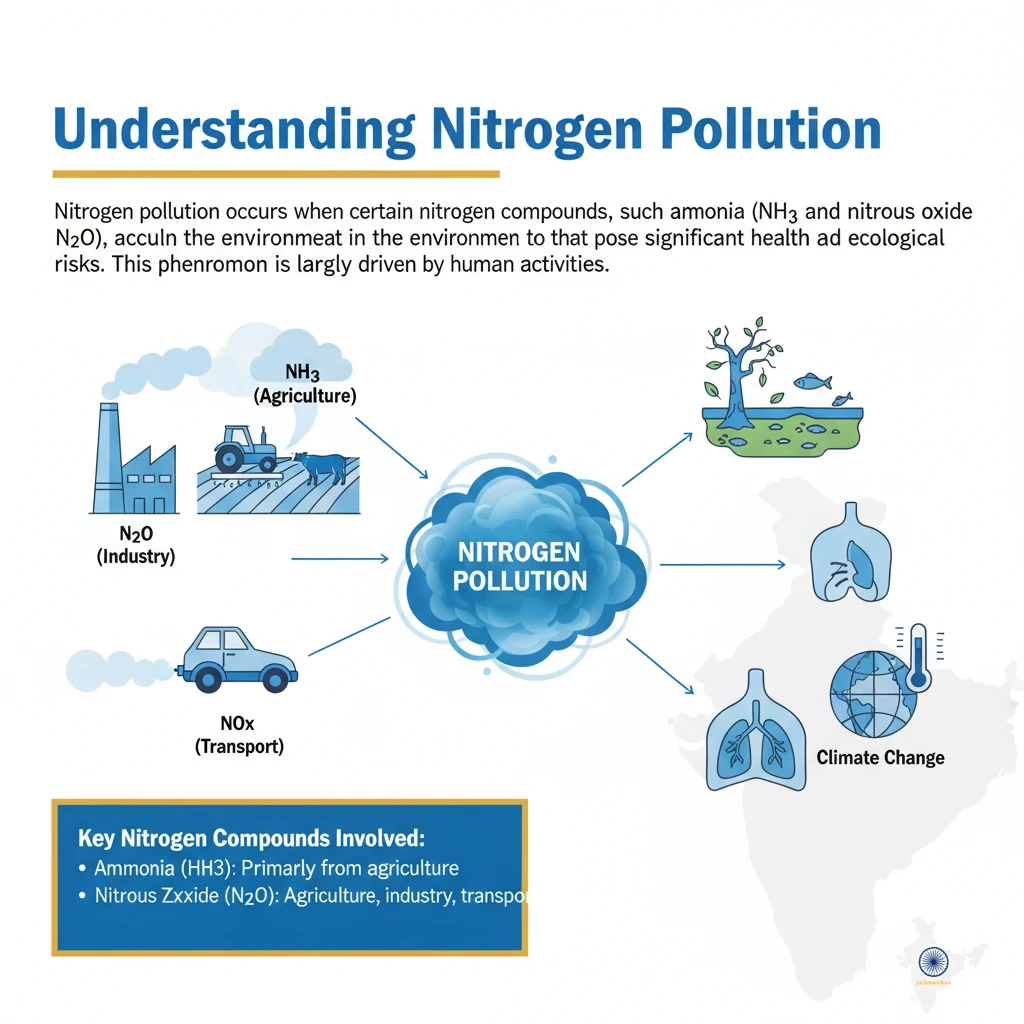
What is Nitrogen Pollution?
Medium⏱️ 10 min read
science and technology
📖 Introduction
<h4>Understanding Nitrogen Pollution</h4><p><strong>Nitrogen pollution</strong> occurs when certain <strong>nitrogen compounds</strong>, such as <strong>ammonia</strong> (NH3) and <strong>nitrous oxide</strong> (N2O), accumulate in the environment to levels that pose significant health and ecological risks. This phenomenon is largely driven by human activities.</p><div class='info-box'><p><strong>Key Nitrogen Compounds Involved:</strong><ul><li><strong>Ammonia (NH3):</strong> Primarily from agricultural sources (fertilizers, livestock waste).</li><li><strong>Nitrous Oxide (N2O):</strong> A potent greenhouse gas and ozone-depleting substance, mainly from agriculture and industrial processes.</li><li><strong>Nitrogen Oxides (NOx):</strong> From combustion processes (vehicles, power plants), contributing to smog.</li></ul></p></div><h4>Human Impact on Nitrogen Cycle</h4><p>Over the past <strong>150 years</strong>, human activities have dramatically altered the natural <strong>nitrogen cycle</strong>. The flow of <strong>reactive nitrogen</strong> – forms of nitrogen that can easily react with other substances – has increased tenfold.</p><p>This surge leads to a dangerous accumulation of unused <strong>reactive nitrogen</strong> within various ecosystems. The natural capacity of the environment to process and cycle nitrogen has been overwhelmed.</p><h4>Agricultural Contribution and Environmental Loss</h4><p>Agriculture is a major contributor to <strong>nitrogen pollution</strong>. The uptake of nitrogen from synthetic fertilizers by crops is often limited and inefficient.</p><p>Each year, an estimated <strong>200 million tonnes</strong> of <strong>reactive nitrogen</strong>, representing about <strong>80%</strong> of applied nitrogen, is lost to the environment. This loss occurs through various pathways.</p><ul><li><strong>Leaching:</strong> Nitrogen compounds seep into <strong>soil</strong>, <strong>rivers</strong>, and <strong>lakes</strong>.</li><li><strong>Emission:</strong> Gaseous nitrogen compounds are released into the <strong>air</strong>.</li></ul><h4>Widespread Environmental and Health Effects</h4><p>The widespread loss of reactive nitrogen results in severe environmental consequences. Ecosystems become <strong>over-enriched</strong>, leading to imbalances and degradation.</p><p>This pollution also contributes to a significant loss of <strong>biodiversity</strong> and directly impacts <strong>human health</strong>. Furthermore, some forms of nitrogen pollution play a role in <strong>ozone depletion</strong> and <strong>climate change</strong>.</p><div class='key-point-box'><p><strong>Overall Impacts:</strong><ul><li><strong>Ecosystem Over-enrichment:</strong> Excess nutrients disrupt natural balances.</li><li><strong>Biodiversity Loss:</strong> Sensitive species are outcompeted.</li><li><strong>Human Health Impacts:</strong> Respiratory issues, water contamination.</li><li><strong>Climate Change:</strong> Potent greenhouse gas emissions.</li><li><strong>Ozone Depletion:</strong> Nitrous oxide is a major threat.</li></ul></p></div><h4>Climate Change and Ozone Layer Impact</h4><p><strong>Nitrous oxide (N2O)</strong> is a particularly concerning nitrogen compound. It is a powerful <strong>greenhouse gas</strong>, approximately <strong>300 times more potent</strong> than both methane and carbon dioxide in terms of its global warming potential over a 100-year period.</p><p>Beyond its role in climate change, <strong>nitrous oxide</strong> is also recognized as the <strong>biggest human-made threat to the ozone layer</strong>. It depletes stratospheric ozone, which protects Earth from harmful ultraviolet radiation.</p><div class='exam-tip-box'><p><strong>UPSC Relevance:</strong> Questions on <strong>greenhouse gases</strong>, <strong>ozone depletion</strong>, and their anthropogenic sources are common in <strong>GS Paper 3 (Environment)</strong>. Understanding N2O's dual impact is crucial.</p></div><h4>Biodiversity and Ecosystem Degradation</h4><p>Nitrogen pollution has profound effects on <strong>biodiversity</strong> and the health of <strong>ecosystems</strong>. It can significantly degrade the quality of soils.</p><p>Excessive application of <strong>synthetic fertilizers</strong>, rich in nitrogen, leads to soil acidification. This damages overall <strong>soil health</strong> and subsequently reduces the <strong>productivity of agricultural lands</strong>.</p><p>In natural environments, nitrogen pollution can cause the inadvertent fertilization of trees and grasslands. This can lead to <strong>nitrogen-tolerant species</strong> outcompeting and displacing more sensitive <strong>wild plants and fungi</strong>, thereby reducing overall biodiversity.</p><p>In aquatic environments, nitrogen pollution is a primary cause of <strong>“dead zones”</strong> in oceans. These are areas with extremely low oxygen levels that cannot support marine life. It also triggers the spread of <strong>toxic algal blooms</strong> in marine ecosystems, harming aquatic life and potentially human health.</p><h4>Air Quality Deterioration</h4><p>Nitrogen compounds significantly contribute to <strong>air pollution</strong>. <strong>Nitrogen oxides (NOx)</strong>, generated from sources like <strong>coal power plants</strong>, <strong>factory emissions</strong>, and <strong>vehicle exhausts</strong>, are key precursors to the formation of <strong>smog</strong> and harmful <strong>ground-level ozone</strong>.</p><p>A particularly dangerous combination arises from <strong>agricultural ammonia emissions</strong>. When these combine with pollution from <strong>vehicle exhausts</strong>, they form extremely hazardous <strong>particulate matter</strong> in the air.</p><p>This fine particulate matter can penetrate deep into the lungs, exacerbating various <strong>respiratory diseases</strong> and posing a serious public health threat.</p>

💡 Key Takeaways
- •Nitrogen pollution is caused by excess reactive nitrogen compounds (ammonia, N2O, NOx) from human activities.
- •Human-driven reactive nitrogen flows have increased tenfold in 150 years, largely due to agriculture.
- •80% of applied nitrogen fertilizer is lost to the environment, leading to over-enrichment and pollution.
- •Nitrous oxide is a potent greenhouse gas (300x CO2) and the biggest human-made threat to the ozone layer.
- •Nitrogen pollution degrades soil, causes biodiversity loss, creates ocean 'dead zones,' and contributes to smog and respiratory diseases.
🧠 Memory Techniques

95% Verified Content
📚 Reference Sources
•UN Environment Programme (UNEP) reports on Nitrogen Pollution
•Intergovernmental Panel on Climate Change (IPCC) reports on N2O