Typbar Typhoid Vaccine - Science And Technology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
Typbar Typhoid Vaccine
Medium⏱️ 8 min read
science and technology
📖 Introduction
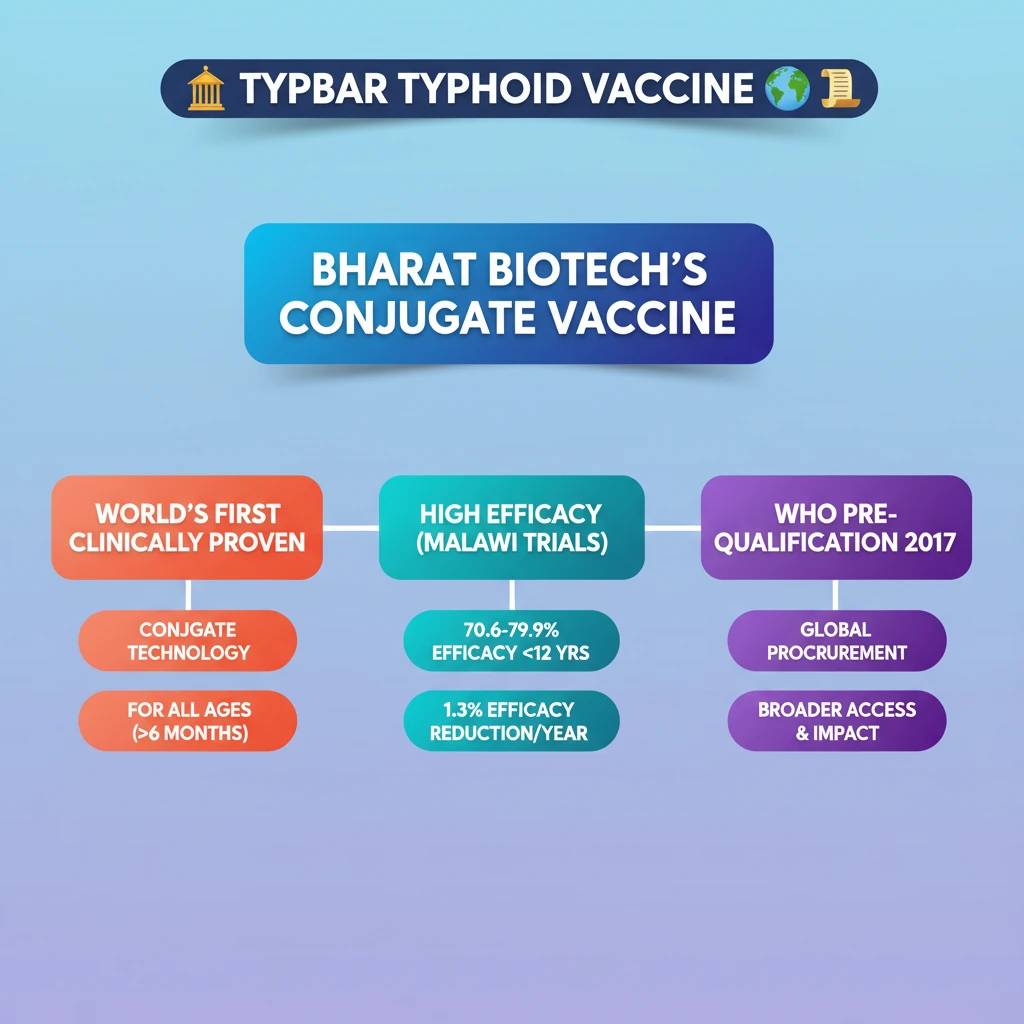
<h4>Introduction to Typbar Typhoid Vaccine</h4><p>The <strong>Typbar Typhoid Vaccine</strong>, developed by <strong>Bharat Biotech</strong>, has recently shown promising <strong>long-term efficacy</strong> in its <strong>Phase-3 trial</strong>.</p><p>This trial was conducted in <strong>Malawi, Africa</strong>, a region significantly affected by <strong>typhoid fever</strong>, demonstrating its effectiveness across various pediatric age groups.</p><div class='key-point-box'><p><strong>Typbar TCV</strong> holds the distinction of being the <strong>world's first clinically proven conjugate Typhoid vaccine</strong>.</p></div><div class='info-box'><p><strong>Note:</strong> <strong>NexCAR19</strong> is the first <strong>CAR-T cell therapy</strong> to receive <strong>CDSCO approval</strong> in India.</p></div><h4>Understanding Conjugate Vaccines</h4><p>A <strong>conjugate vaccine</strong> is a sophisticated type of vaccine designed to enhance the immune response against certain pathogens.</p><div class='info-box'><p>It functions by combining a <strong>weak antigen</strong> with a <strong>strong antigen</strong>, often referred to as a <strong>carrier protein</strong>.</p></div><p>This strategic combination enables the immune system to mount a much <strong>stronger and more effective immune response</strong> to the otherwise weak antigen.</p><p>The result is enhanced protection against infections caused by the pathogen from which the weak antigen originated.</p><h4>Key Findings from Typbar Vaccine Trials</h4><p>The <strong>Phase-3 trial</strong> for <strong>Typbar TCV</strong> yielded significant results, particularly concerning its protective capabilities in children.</p><p>The trial demonstrated the vaccine's efficacy in children across <strong>all age groups under 12 years</strong>.</p><div class='info-box'><p>The trial took place in <strong>Malawi, Africa</strong>, a setting where <strong>typhoid fever</strong> is endemic, involving children aged <strong>nine months to 12 years</strong>.</p></div><p>A single dose of the vaccine was administered to children between <strong>February and September 2018</strong>.</p><ul><li><strong>Vaccine Group:</strong> <strong>14,069 children</strong> received the <strong>typhoid vaccine</strong>.</li><li><strong>Control Group:</strong> <strong>14,061 children</strong> received a <strong>control vaccine (Mena)</strong>.</li></ul><div class='key-point-box'><p>The <strong>efficacy</strong> at the end of a <strong>4.3-year median follow-up</strong> period was notably high across different age brackets.</p></div><ul><li><strong>Efficacy (9 months to 2 years):</strong> <strong>70.6%</strong></li><li><strong>Efficacy (2-4 years):</strong> <strong>79.6%</strong></li><li><strong>Efficacy (5-12 years):</strong> <strong>79.9%</strong></li></ul><p>The trial also revealed an <strong>absolute risk reduction</strong> of <strong>6.1 typhoid infections per 1,000 vaccinated children</strong>.</p><p>Furthermore, the estimated reduction in vaccine efficacy over time was minimal, only <strong>1.3% per year over four years</strong>.</p><div class='info-box'><p><strong>Typbar TCV</strong>, manufactured by <strong>Bharat Biotech</strong>, received <strong>WHO prequalification in 2017</strong>, marking a significant global recognition.</p></div><h4>What is Typhoid?</h4><p><strong>Typhoid fever</strong> is a severe and potentially life-threatening infection.</p><div class='info-box'><p>It is caused by the bacterium <strong><em>Salmonella Typhi</em></strong>.</p></div><p>The primary mode of transmission for typhoid is typically through the consumption of <strong>contaminated food or water</strong>.</p>
💡 Key Takeaways
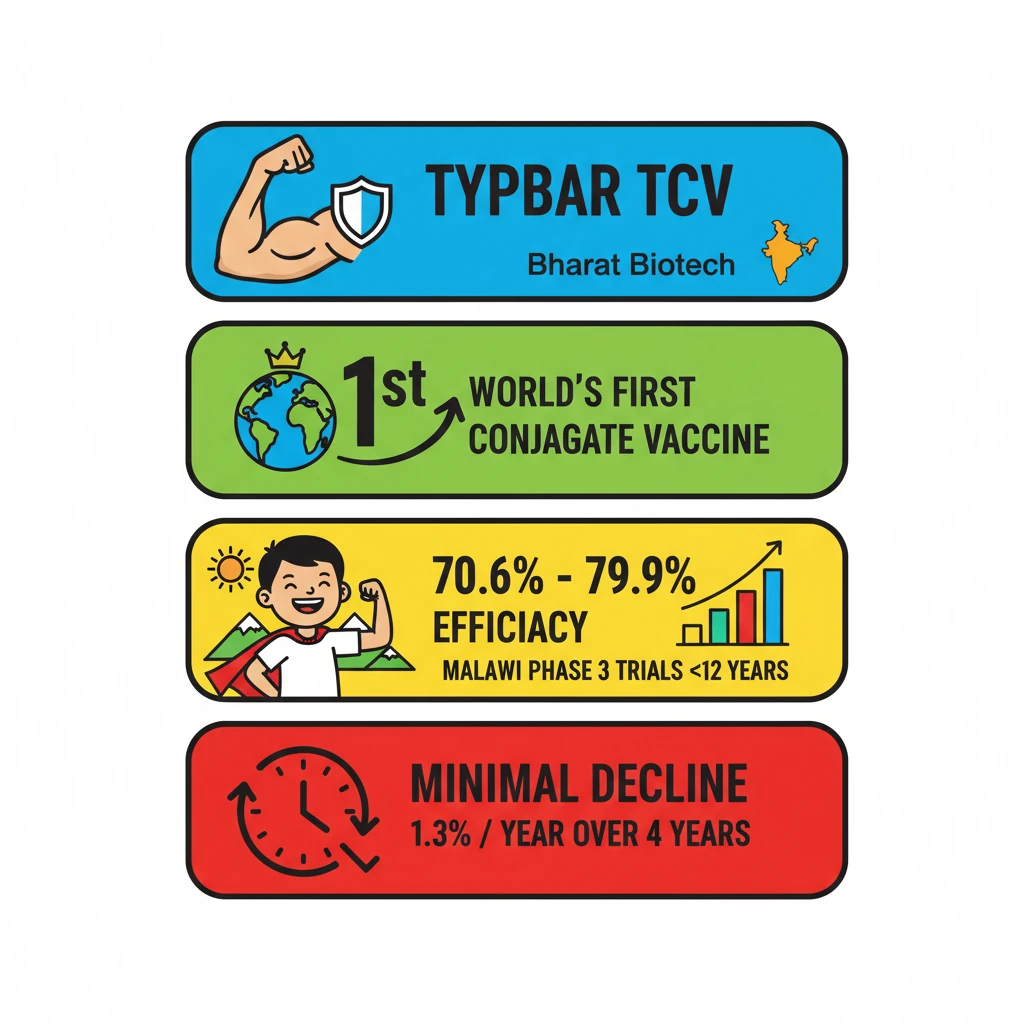
- •Typbar TCV is Bharat Biotech's conjugate typhoid vaccine.
- •It's the world's first clinically proven conjugate typhoid vaccine.
- •Phase-3 trials in Malawi showed high long-term efficacy (70.6% to 79.9%) in children under 12.
- •Efficacy reduction over time is minimal (1.3% per year over four years).
- •WHO prequalification in 2017 enables global procurement.
- •Typhoid fever is caused by Salmonella Typhi, spread via contaminated food/water.
🧠 Memory Techniques
95% Verified Content