What We Lose With Age - Science And Technology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
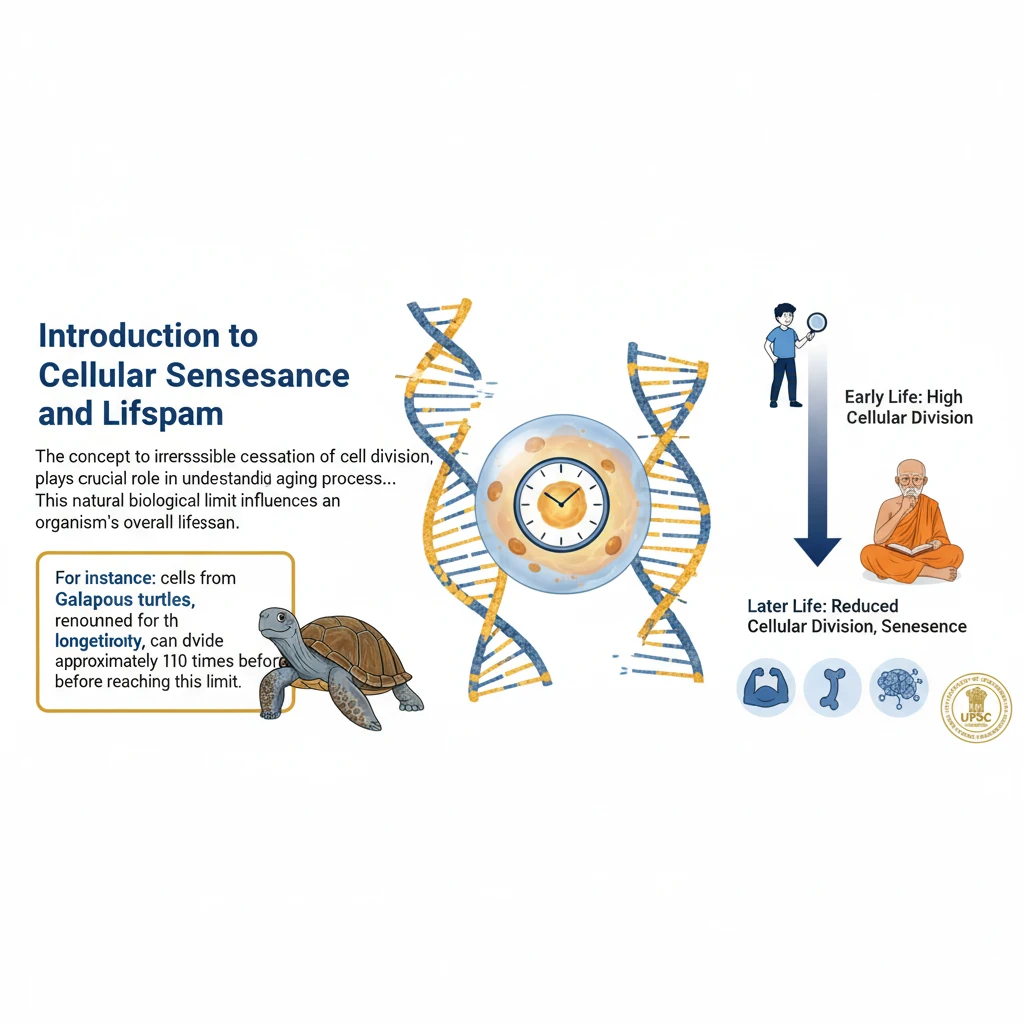
What We Lose With Age
Medium⏱️ 4 min read
science and technology
📖 Introduction
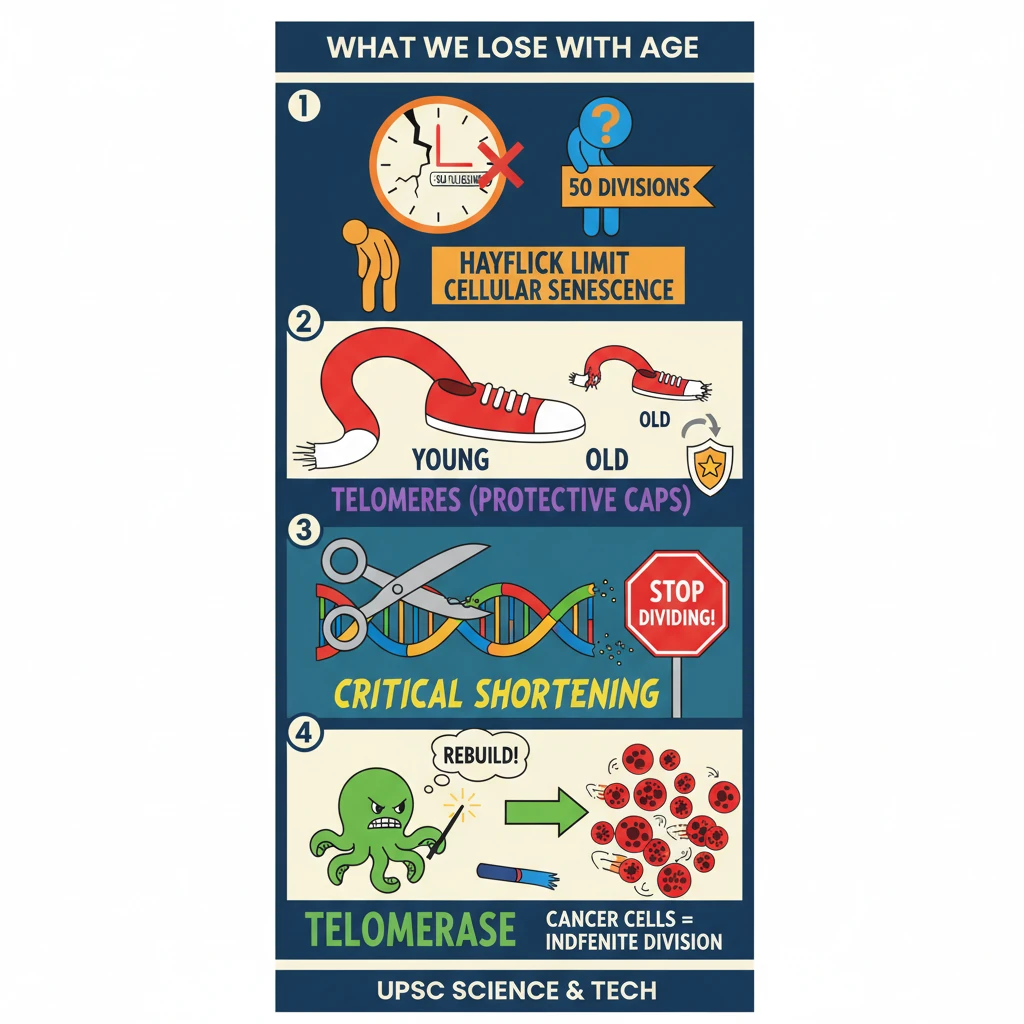
<h4>Introduction to Cellular Senescence and Lifespan</h4><p>The concept of <strong>cellular senescence</strong>, or the irreversible cessation of cell division, plays a crucial role in understanding the aging process across different species. This natural biological limit influences an organism's overall lifespan.</p><div class='info-box'><p>For instance, cells from <strong>Galapagos turtles</strong>, renowned for their longevity, can divide approximately <strong>110 times</strong> before reaching senescence. This correlates with their remarkable lifespan of over <strong>200 years</strong>.</p><p>In stark contrast, cells from typical <strong>laboratory mice</strong> become senescent after only about <strong>15 divisions</strong>. This significantly lower number aligns with their much shorter lifespans.</p></div><h4>The Role of Telomeres in Cell Division</h4><p>Further scientific investigations in the <strong>1970s</strong> led to a significant discovery: <strong>telomeres</strong>. These are specialized, repetitive sequences of <strong>Deoxyribonucleic acid (DNA)</strong> found at the very ends of <strong>chromosomes</strong>.</p><div class='key-point-box'><p>The primary function of <strong>telomeres</strong> is to protect the genetic information contained within <strong>chromosomes</strong> during the process of <strong>cell division</strong>. They act like caps, preventing the loss of vital DNA sequences.</p></div><p>However, with each successive round of <strong>cell division</strong>, <strong>telomeres</strong> progressively become shorter. This shortening continues until they reach a <strong>critical length</strong>, which then signals the cell to stop dividing.</p><p>This mechanism of <strong>telomere shortening</strong> is widely understood to be a significant contributor to the phenomenon of <strong>ageing</strong> and the eventual decline of cellular function.</p><h4>Telomere Length: A Complex Indicator of Lifespan</h4><p>While a clear link exists between <strong>telomere shortening</strong> and the process of <strong>ageing</strong>, the precise correlation between an organism's initial <strong>telomere length</strong> and its ultimate <strong>lifespan</strong> is not straightforward or absolute.</p><div class='info-box'><p>A notable example is the comparison between humans and mice. Despite having significantly longer <strong>telomeres</strong> than humans, <strong>mice</strong> exhibit considerably shorter lifespans. This suggests other complex factors are at play.</p></div><p>Some researchers propose that <strong>telomere loss</strong> and the <strong>Hayflick limit</strong> (the finite number of times a normal human cell population will divide in vitro) may not be direct causes of <strong>ageing</strong>. Instead, they might be considered symptoms or manifestations of the broader <strong>ageing process</strong> itself.</p><h4>Telomerase and the Hayflick Limit</h4><p>In the <strong>1980s</strong>, scientists made another groundbreaking discovery: a protein called <strong>telomerase</strong>. This remarkable enzyme possesses the unique ability to produce new <strong>telomeres</strong>, effectively counteracting the shortening process.</p><div class='key-point-box'><p><strong>Telomerase</strong> is notably active in <strong>cancer cells</strong>. Its presence allows these cells to bypass the natural <strong>Hayflick limit</strong>, enabling them to continue dividing indefinitely and contributing to uncontrolled growth.</p></div><p>As famously stated by <strong>Hayflick</strong> himself, this is precisely why <strong>cancer cells</strong> are not subject to the normal limitations imposed by the <strong>Hayflick Limit</strong>, allowing them to achieve immortality in a laboratory setting.</p><h4>Challenges in Telomerase Application for Anti-Aging</h4><p>Despite its potential, the primary activity of <strong>telomerase</strong> in <strong>cancer cells</strong> presents significant challenges for its therapeutic application in healthy cells. Harnessing its power without risking cancerous growth is a major hurdle.</p><p>Although scientists have successfully synthesized <strong>telomerase</strong> in laboratories, and some <strong>in vitro studies</strong> have shown promise in slowing down <strong>telomere loss</strong> in normal human cells, practical application remains a distant goal.</p><div class='exam-tip-box'><p>Understanding the dual nature of <strong>telomerase</strong> – its role in both <strong>cancer</strong> and potential <strong>anti-aging therapies</strong> – is crucial for <strong>UPSC Mains GS Paper 3</strong> questions on <strong>biotechnology</strong> and <strong>health research</strong>. Focus on the ethical and practical challenges.</p></div>
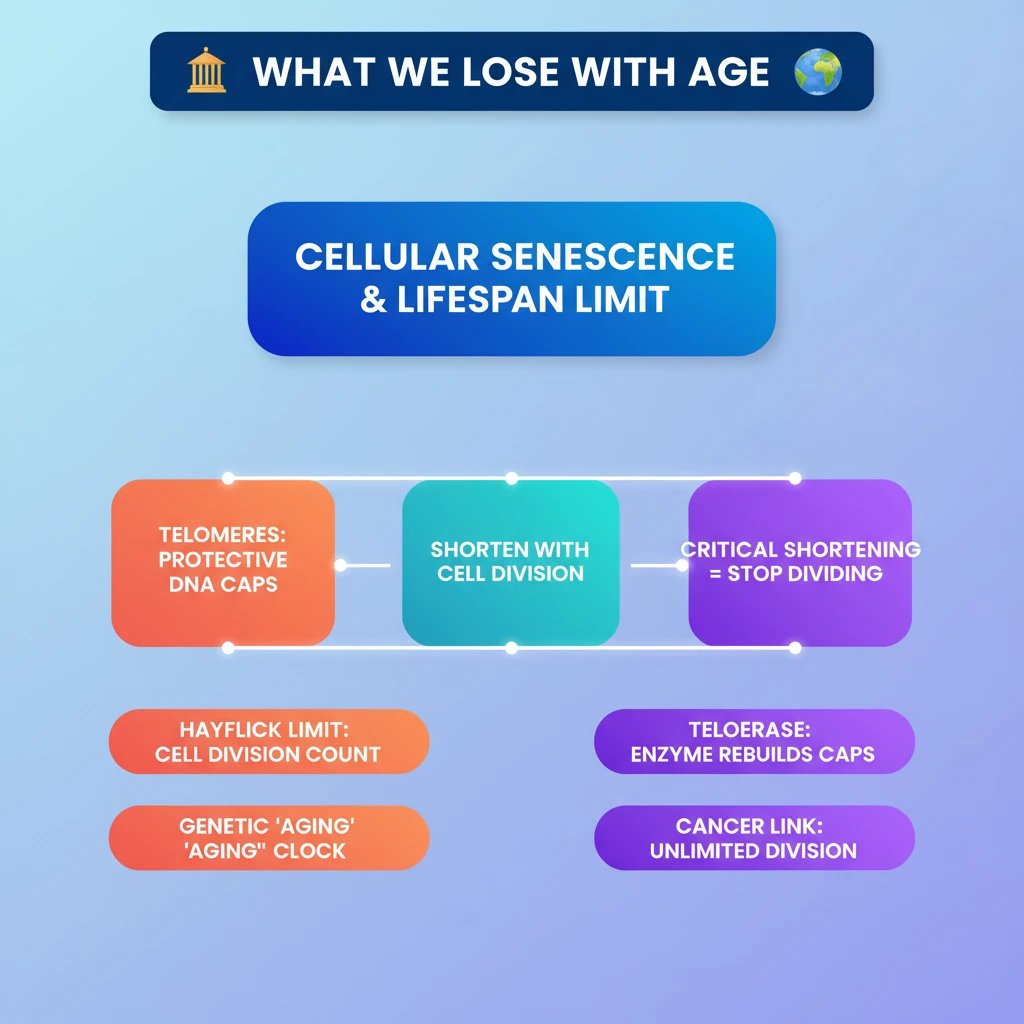
💡 Key Takeaways
- •Cellular senescence (Hayflick Limit) dictates how many times cells divide, influencing species lifespan.
- •Telomeres are protective DNA caps on chromosomes that shorten with each cell division.
- •Critical telomere shortening signals cells to stop dividing, contributing to aging.
- •Telomerase is an enzyme that can rebuild telomeres, active in cancer cells allowing indefinite division.
- •While telomerase holds anti-aging potential, its link to cancer complicates therapeutic application.
🧠 Memory Techniques
95% Verified Content
📚 Reference Sources
•General scientific literature on telomeres, telomerase, and cellular senescence