What is Nitrogen Pollution? - Environment And Ecology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
What is Nitrogen Pollution?
Medium⏱️ 8 min read
environment and ecology
📖 Introduction
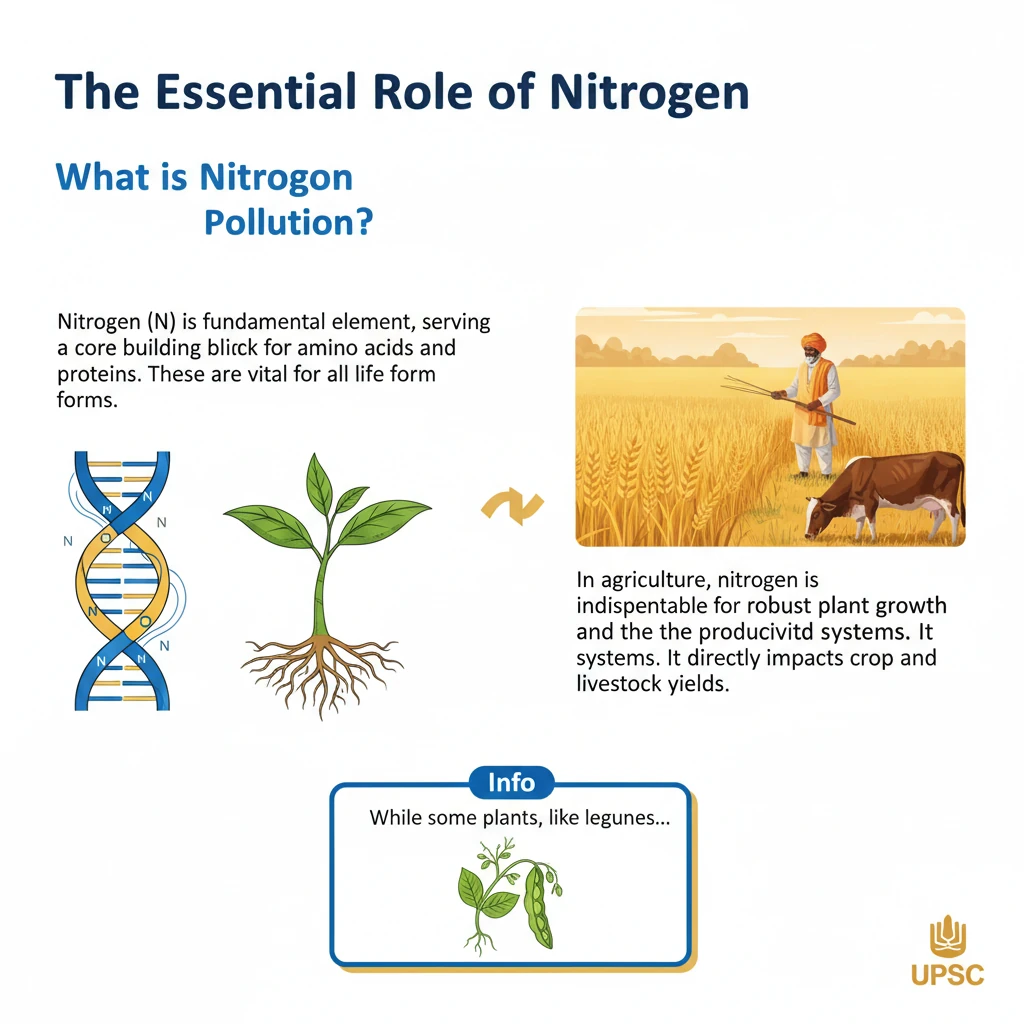
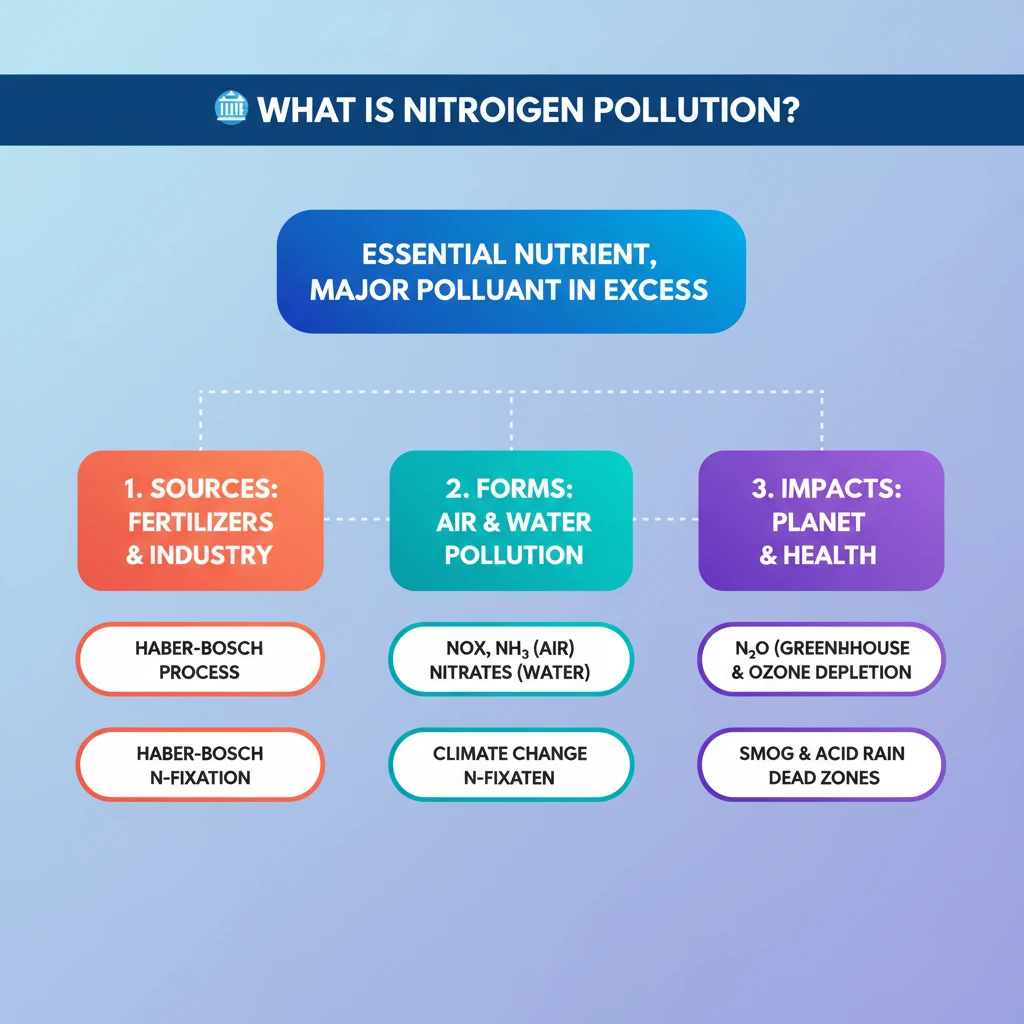
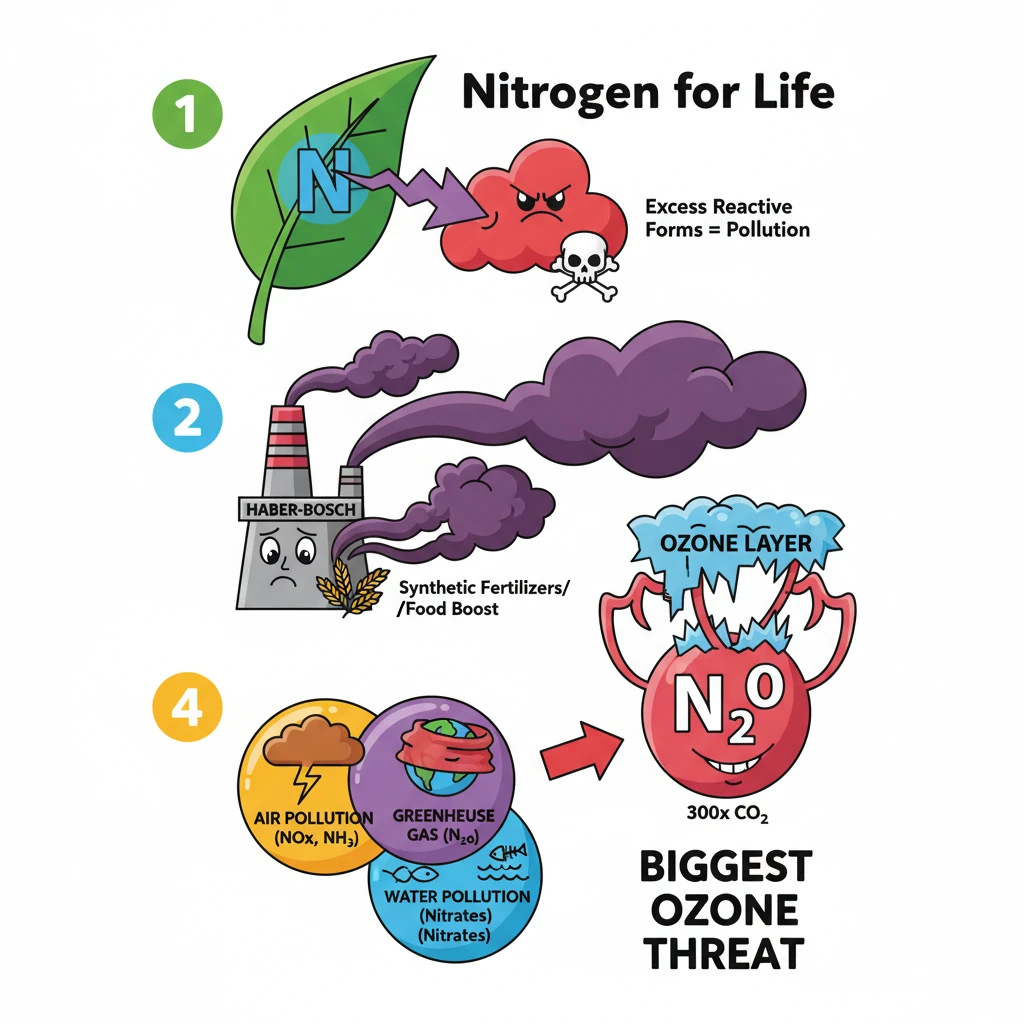
<h4>The Essential Role of Nitrogen</h4><p><strong>Nitrogen (N)</strong> is a fundamental element, serving as a core building block for <strong>amino acids</strong> and <strong>proteins</strong>. These are vital for all life forms.</p><p>In agriculture, nitrogen is indispensable for robust <strong>plant growth</strong> and the productivity of <strong>agrifood systems</strong>. It directly impacts crop and livestock yields.</p><div class='info-box'><p>While some plants, like <strong>legumes</strong>, can fix atmospheric nitrogen, most depend heavily on <strong>soil nitrogen</strong> for their nutritional needs.</p></div><h4>The Haber-Bosch Process and Reactive Nitrogen</h4><p>The development of the <strong>Haber-Bosch process</strong> revolutionized agriculture. This industrial method converts inert atmospheric nitrogen (N₂) into highly <strong>reactive nitrogen</strong> compounds, primarily <strong>ammonium (NH₃)</strong>.</p><p>This process enabled the widespread production of <strong>synthetic fertilizers</strong>, which significantly boosted global crop production and food security.</p><div class='key-point-box'><p>The conversion of inert nitrogen into reactive forms is crucial for plant uptake but also a primary driver of nitrogen pollution.</p></div><h4>Understanding Nitrogen Pollution</h4><p><strong>Nitrogen pollution</strong> refers to the excessive accumulation of nitrogen compounds in the environment. These compounds primarily include <strong>nitrogen oxides (NOx)</strong> and <strong>nitrates (NO₃⁻)</strong>.</p><p>Such excessive presence disrupts natural biogeochemical cycles and leads to widespread environmental degradation.</p><div class='info-box'><p>The loss of reactive nitrogen to the environment has detrimental effects on <strong>air quality</strong>, <strong>water quality</strong>, <strong>human health</strong>, and <strong>biodiversity</strong> across both terrestrial and aquatic ecosystems.</p></div><h4>Forms of Environmental Nitrogen Loss</h4><p>Nitrogen pollution manifests in various forms, impacting different environmental compartments:</p><ul><li><strong>Air Pollution:</strong> Emissions of <strong>ammonia (NH₃)</strong> from agriculture and <strong>nitrogen oxides (NOx)</strong> from combustion sources contribute significantly to air quality degradation.</li><li><strong>Greenhouse Gas Emissions:</strong> <strong>Nitrous oxide (N₂O)</strong> is a potent <strong>greenhouse gas (GHG)</strong>, playing a substantial role in accelerating <strong>climate change</strong>.</li><li><strong>Water Pollution:</strong> The leaching of <strong>nitrates (NO₃⁻)</strong> into water bodies causes <strong>eutrophication</strong> and <strong>acidification</strong>, severely harming aquatic ecosystems and diminishing water quality.</li></ul><h4>Escalating Concerns: The Scale of Nitrogen Pollution</h4><p>Over the past <strong>150 years</strong>, human activities have dramatically increased the flow of <strong>reactive nitrogen</strong> into the environment, estimated to have risen tenfold.</p><p>Annually, approximately <strong>200 million tonnes</strong> of reactive nitrogen are lost, with about <strong>80%</strong> of this contaminating soil, rivers, lakes, and the atmosphere.</p><div class='exam-tip-box'><p>UPSC often asks about the scale and human impact on biogeochemical cycles. Remember the <strong>tenfold increase</strong> and <strong>200 million tonnes annual loss</strong> as key figures.</p></div><h4>Multi-faceted Environmental Impacts</h4><h5>Global Warming and Ozone Layer Depletion</h5><p><strong>Nitrous oxide (N₂O)</strong> is a particularly potent greenhouse gas, approximately <strong>300 times more powerful</strong> than both methane and carbon dioxide in its warming potential.</p><p>Furthermore, N₂O represents the <strong>largest human-made threat</strong> to the Earth's protective <strong>ozone layer</strong>, contributing to its depletion.</p><h5>Impact on Biodiversity</h5><p>Excessive use of <strong>synthetic fertilizers</strong> can degrade soils by making them <strong>acidic</strong>. This harms <strong>soil health</strong>, reduces microbial activity, and diminishes agricultural productivity.</p><p>In aquatic environments, nitrogen pollution leads to the formation of <strong>dead zones</strong> in oceans and fuels the proliferation of <strong>toxic algal blooms</strong>, severely impacting marine ecosystems and biodiversity.</p><h5>Air Quality Degradation</h5><p>Emissions of <strong>nitrogen oxides (NOx)</strong> from sources like <strong>coal plants</strong>, <strong>factories</strong>, and <strong>vehicle exhausts</strong> are primary precursors to <strong>smog</strong> and the formation of harmful <strong>ground-level ozone</strong>.</p><p>Additionally, <strong>agricultural ammonia</strong> and vehicle exhaust emissions contribute to the creation of fine <strong>particulates</strong>, which exacerbate respiratory diseases and other health issues in humans.</p>
💡 Key Takeaways
- •Nitrogen is essential for life but its reactive forms, when in excess, cause significant pollution.
- •The Haber-Bosch process enabled synthetic fertilizers, boosting food but also reactive nitrogen flows.
- •Nitrogen pollution manifests as air pollution (NOx, NH₃), greenhouse gas (N₂O), and water pollution (nitrates).
- •Nitrous oxide (N₂O) is a powerful GHG (300x CO₂) and the largest human threat to the ozone layer.
- •Effects include global warming, ozone depletion, biodiversity loss (dead zones, acidic soils), and severe air quality degradation (smog, particulates).
🧠 Memory Techniques
95% Verified Content
📚 Reference Sources
•General environmental science textbooks and reports on nitrogen cycle and pollution.