Formation of Acid Rain - Environment And Ecology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search

Formation of Acid Rain
Easy⏱️ 5 min read
environment and ecology
📖 Introduction
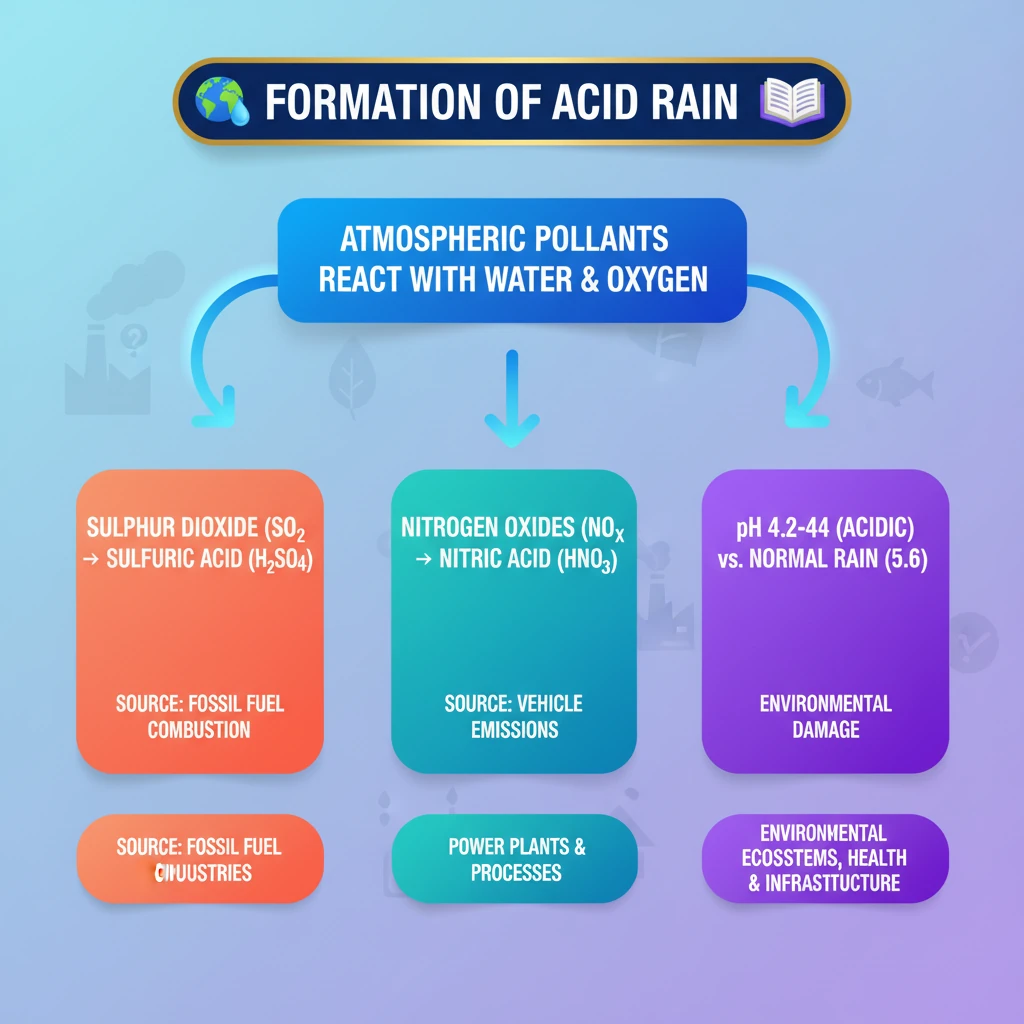
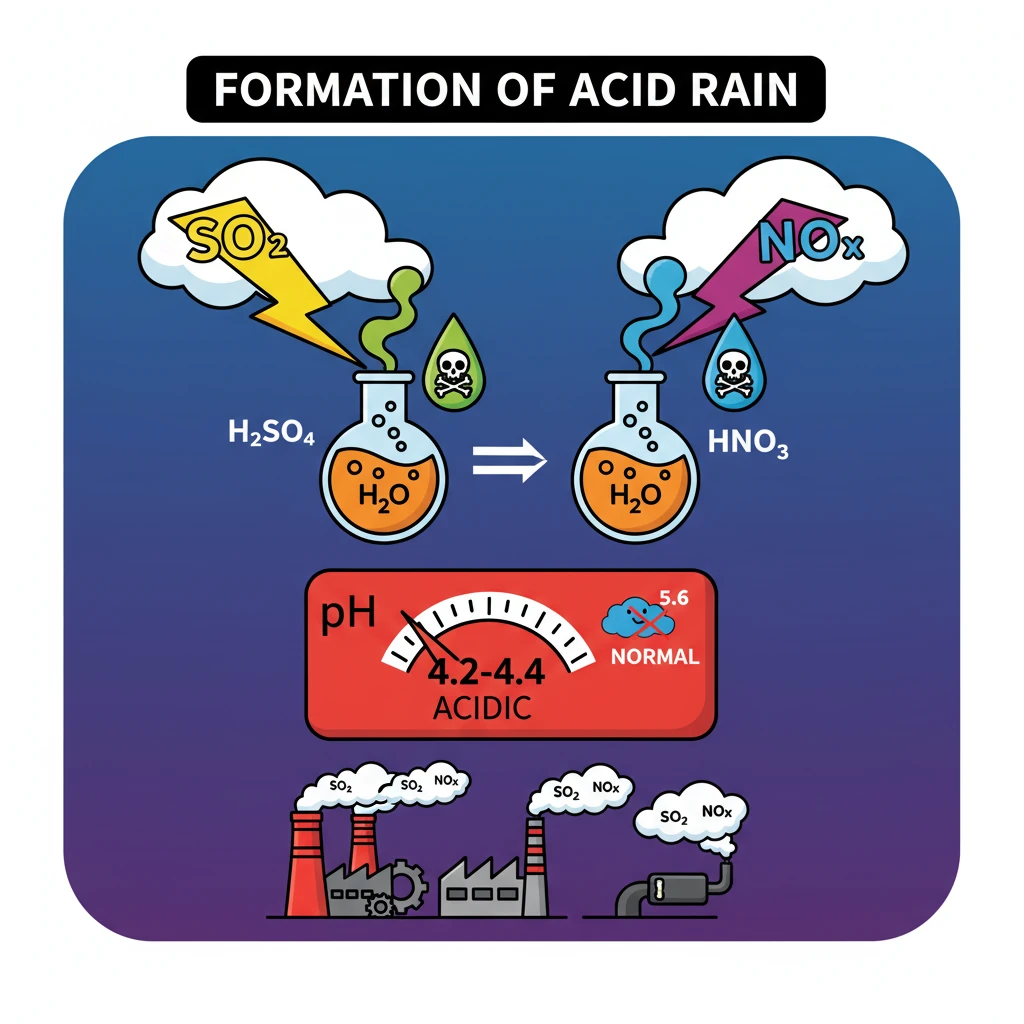
<h4>Understanding Acid Rain Formation</h4><p><strong>Acid rain</strong> refers to any form of precipitation with high levels of <strong>nitric</strong> and <strong>sulfuric acids</strong>. It can also occur in the form of snow, fog, or even dry particles.</p><p>This phenomenon is primarily caused by atmospheric pollution, specifically the emission of certain gases into the atmosphere.</p><h4>Primary Pollutants: Sulphur Dioxide and Nitrogen Oxides</h4><p>The two main gaseous pollutants responsible for acid rain formation are <strong>Sulphur Dioxide (SO2)</strong> and <strong>Nitrogen Oxides (NOx)</strong>. These gases are released into the atmosphere from various sources.</p><div class='info-box'><ul><li><strong>Sulphur Dioxide (SO2):</strong> Primarily originates from the burning of fossil fuels (coal, oil) in power plants and industrial facilities.</li><li><strong>Nitrogen Oxides (NOx):</strong> Produced from vehicle exhausts, industrial combustion processes, and power generation.</li></ul></div><h4>Atmospheric Chemical Reactions</h4><p>Once released, <strong>SO2</strong> and <strong>NOx</strong> do not immediately form acid rain. They undergo a series of complex chemical reactions in the atmosphere.</p><div class='key-point-box'><p>These gases combine with <strong>water vapor (H2O)</strong> and <strong>oxygen (O2)</strong> present in the atmosphere. This oxidative process transforms them into their acidic forms.</p></div><p>Specifically, <strong>Sulphur Dioxide (SO2)</strong> reacts to form <strong>Sulfuric Acid (H2SO4)</strong>, and <strong>Nitrogen Oxides (NOx)</strong> react to form <strong>Nitric Acid (HNO3)</strong>.</p><h4>Formation of Acidic Precipitation</h4><p>The newly formed <strong>sulfuric acid</strong> and <strong>nitric acid</strong> then dissolve into the tiny water droplets within clouds.</p><p>As these water droplets grow and condense, they fall to the Earth's surface as <strong>acid rain</strong>, <strong>acid snow</strong>, or <strong>acid fog</strong>, depending on atmospheric conditions.</p><h4>Understanding pH Levels</h4><p>The acidity of precipitation is measured using the <strong>pH scale</strong>. A lower pH indicates higher acidity.</p><div class='info-box'><ul><li>The typical <strong>pH</strong> of <strong>normal rain</strong> is around <strong>5.6</strong>. This slight acidity is due to the natural presence of carbon dioxide in the atmosphere forming carbonic acid.</li><li>In contrast, the <strong>pH</strong> of <strong>acid rain</strong> is significantly lower, typically ranging from <strong>4.2 to 4.4</strong>, indicating its much higher acidic nature.</li></ul></div><div class='exam-tip-box'><p>Understanding the <strong>chemical reactions</strong> and the <strong>pH values</strong> is crucial for UPSC Mains, especially for questions on environmental pollution and its impacts.</p></div>
💡 Key Takeaways
- •Acid rain forms when Sulphur Dioxide (SO2) and Nitrogen Oxides (NOx) react with atmospheric water and oxygen.
- •SO2 primarily forms Sulfuric Acid (H2SO4), and NOx forms Nitric Acid (HNO3).
- •The typical pH of acid rain (4.2-4.4) is significantly more acidic than normal rain (5.6).
- •Major sources of these pollutants are fossil fuel combustion from power plants, industries, and vehicles.
- •Acid rain causes widespread environmental damage to ecosystems, infrastructure, and human health.
🧠 Memory Techniques
95% Verified Content
📚 Reference Sources
•NCERT Class 11 Chemistry (Environmental Chemistry chapter)
•US EPA resources on Acid Rain
•United Nations Environment Programme (UNEP) reports