Acid Rain: Formation, Causes, and Environmental Impacts - Environment And Ecology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search

Acid Rain: Formation, Causes, and Environmental Impacts
Medium⏱️ 8 min read
environment and ecology
📖 Introduction
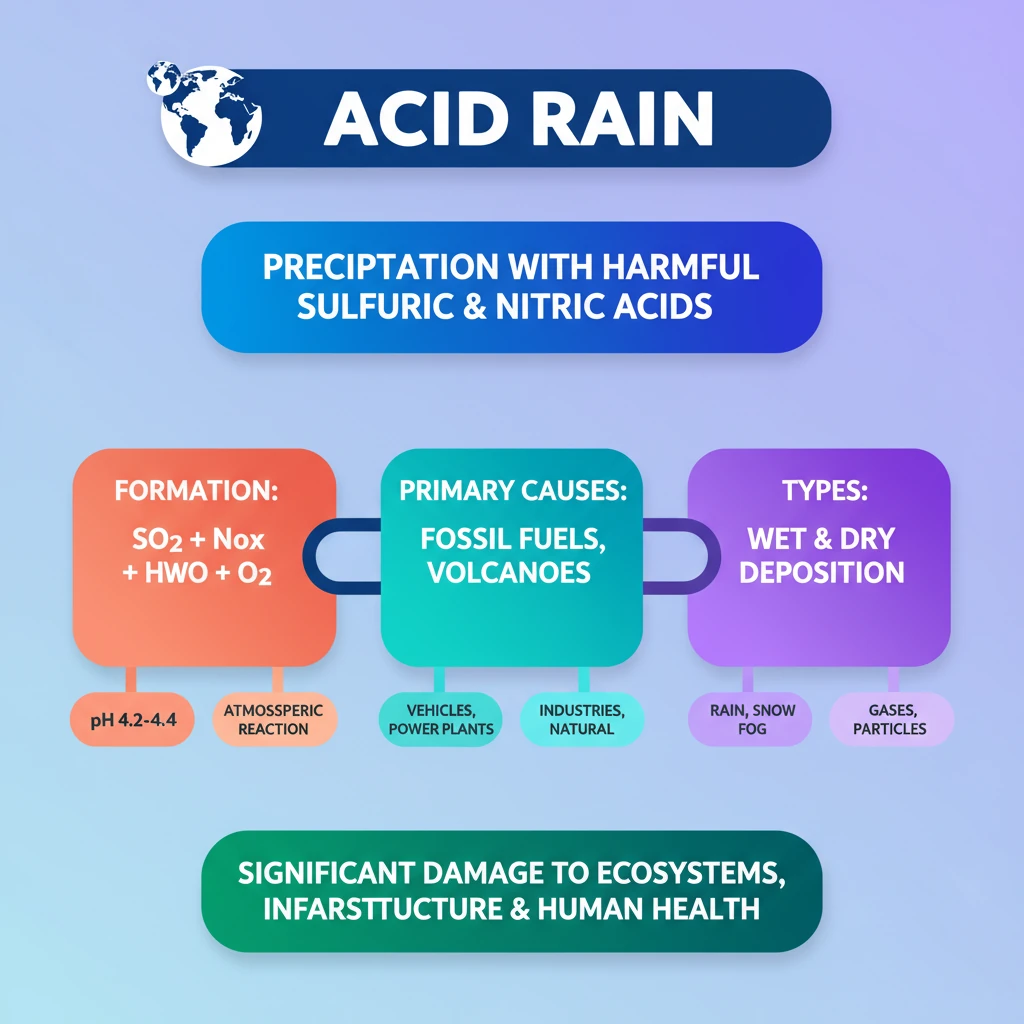
<h4>Understanding Acid Rain</h4><p><strong>Acid Rain</strong>, also known as <strong>acid deposition</strong>, is a broad environmental term.</p><p>It encompasses any form of <strong>precipitation</strong> with acidic components that fall to the ground from the atmosphere.</p><div class='info-box'><p>These acidic components primarily include <strong>sulfuric acid</strong> (H2SO4) and <strong>nitric acid</strong> (HNO3).</p></div><p>It can manifest in various forms such as <strong>rain</strong>, <strong>snow</strong>, <strong>fog</strong>, <strong>hail</strong>, or even acidic <strong>dust</strong>.</p><h4>Formation Process of Acid Rain</h4><p>The formation of acid rain begins with the release of specific atmospheric pollutants.</p><p>Key pollutants are <strong>Sulphur Dioxide</strong> (SO2) and <strong>Nitrogen Oxide</strong> (NOx).</p><div class='key-point-box'><p>When <strong>SO2</strong> and <strong>NOx</strong> combine with <strong>water</strong> and <strong>oxygen</strong> in the atmosphere, they undergo chemical reactions.</p></div><p>These reactions lead to the formation of <strong>sulfuric acid</strong> (H2SO4) from SO2 and <strong>nitric acid</strong> (HNO3) from NOx.</p><p>These newly formed acids then dissolve into atmospheric <strong>water droplets</strong>.</p><p>This dissolution results in the creation of <strong>acid rain</strong>, <strong>acid snow</strong>, or <strong>acid fog</strong>.</p><div class='info-box'><p>The typical <strong>pH</strong> (Potential of Hydrogen) of acid rain ranges from <strong>4.2–4.4</strong>.</p></div><p>This pH value signifies it is considerably more acidic than <strong>normal rain</strong>, which typically has a <strong>pH of around 5.6</strong>.</p><h4>Primary Causes of Acid Rain</h4><p>Acid rain primarily originates from both anthropogenic (human-induced) and natural sources.</p><h5>Anthropogenic Causes: Fossil Fuel Combustion</h5><p>The burning of <strong>Fossil Fuels</strong> is a major contributor, especially those rich in <strong>sulfur</strong>.</p><p>This process releases large quantities of <strong>sulfur dioxide</strong> (SO2) and, at higher temperatures, <strong>nitrogen oxides</strong> (NOx).</p><div class='info-box'><p><strong>Fossil fuel combustion</strong> is widespread in <strong>vehicles</strong> like automobiles, making them a primary source of these pollutants.</p></div><p>The combustion of <strong>coal</strong> in <strong>power plants</strong> and various <strong>industrial processes</strong> also significantly releases these harmful substances into the atmosphere.</p><h5>Natural Sources</h5><p>Certain natural phenomena also contribute to atmospheric <strong>SO2</strong> and <strong>NOx</strong>.</p><p>These include <strong>Volcanic Eruptions</strong>, which release sulfur compounds, and <strong>Lightning</strong>, which can fix atmospheric nitrogen into nitrogen oxides.</p><h5>Atmospheric Air Pollution</h5><p>Once released, <strong>SO2</strong> and <strong>NOx</strong> pollutants undergo complex <strong>chemical reactions</strong> in the atmosphere.</p><p>These reactions transform them into <strong>sulfuric</strong> and <strong>nitric acids</strong>.</p><div class='key-point-box'><p>When these acids combine with <strong>water vapor</strong>, they lead to <strong>acid rain</strong> during precipitation events.</p></div><h4>Forms of Acid Deposition</h4><p>Acid deposition occurs in two primary forms, depending on the presence of moisture.</p><h5>Wet Deposition</h5><p>In <strong>wet deposition</strong>, the <strong>sulfuric</strong> and <strong>nitric acids</strong> formed in the atmosphere fall to the ground.</p><p>They are mixed with various forms of precipitation, including <strong>rain</strong>, <strong>snow</strong>, <strong>fog</strong>, or <strong>hail</strong>.</p><h5>Dry Deposition</h5><p><strong>Dry deposition</strong> involves acidic <strong>particles</strong> and <strong>gases</strong> settling from the atmosphere without the presence of moisture.</p><div class='info-box'><p>These acidic particles and gases can deposit directly onto surfaces such as <strong>water bodies</strong>, <strong>vegetation</strong>, and <strong>buildings</strong>.</p></div><p>Alternatively, they may react during atmospheric transport to form larger particles that can pose risks to <strong>human health</strong>.</p><div class='exam-tip-box'><p>UPSC often asks about the distinction between <strong>wet</strong> and <strong>dry deposition</strong>. Understand both forms and their implications for environmental damage.</p></div>
💡 Key Takeaways
- •Acid rain is precipitation (wet or dry) with high levels of sulfuric and nitric acids, having a pH of 4.2-4.4.
- •It forms when Sulphur Dioxide (SO2) and Nitrogen Oxides (NOx) react with atmospheric water and oxygen.
- •Primary causes include burning fossil fuels (vehicles, power plants, industries) and natural sources like volcanic eruptions and lightning.
- •Acid deposition occurs as wet deposition (rain, snow, fog) or dry deposition (acidic particles/gases).
- •It causes significant damage to ecosystems (forests, aquatic life), infrastructure (historical monuments), and poses risks to human health.
🧠 Memory Techniques

95% Verified Content