Causes of Acid Rain - Environment And Ecology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
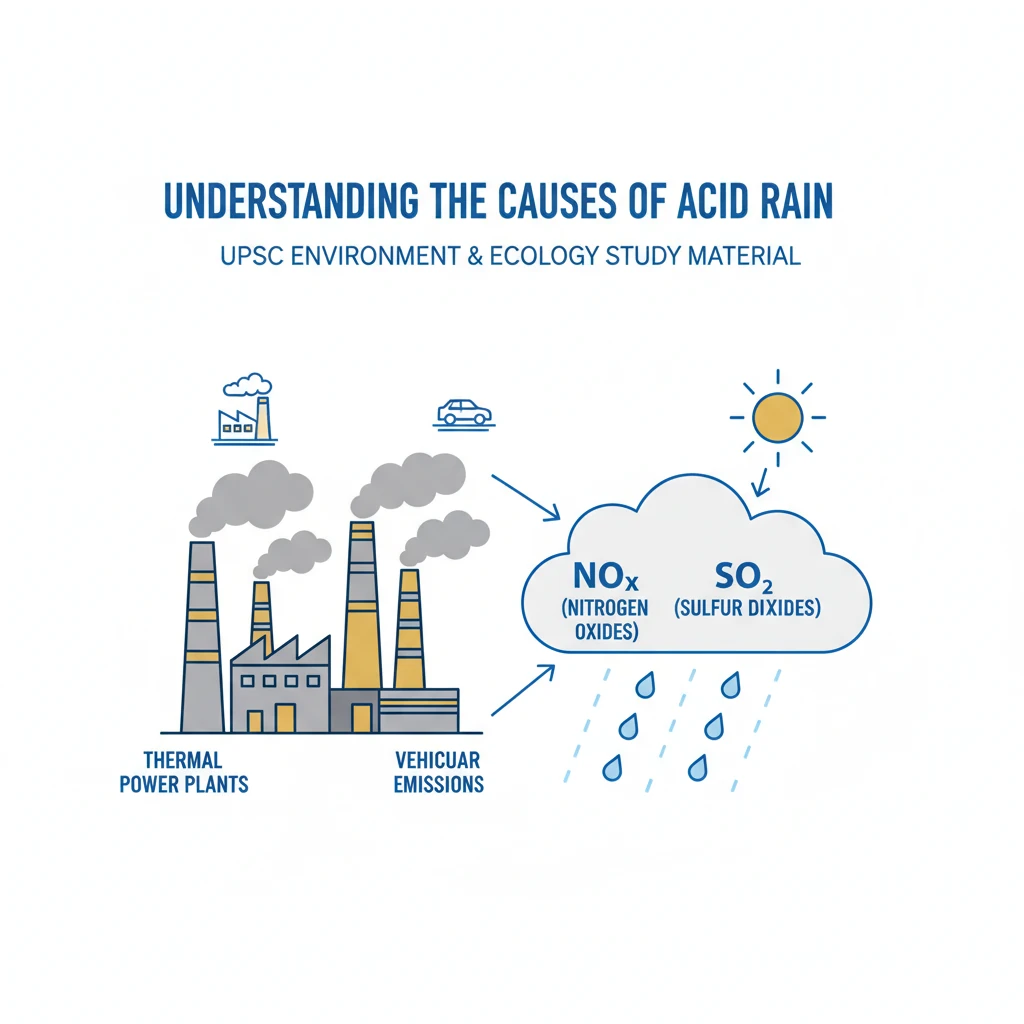
Causes of Acid Rain
Medium⏱️ 8 min read
environment and ecology
📖 Introduction
<h4>Understanding the Causes of Acid Rain</h4><p><strong>Acid rain</strong> refers to any form of precipitation with high levels of <strong>nitric</strong> and <strong>sulfuric acids</strong>. It can also include other forms of acidic deposition such as fog, snow, and dry particulate matter. Understanding its causes is crucial for environmental management.</p><div class='info-box'><p><strong>Definition:</strong> <strong>Acid rain</strong> is precipitation (rain, snow, fog, dry particles) that is more acidic than normal, typically with a <strong>pH less than 5.6</strong>, due to atmospheric pollution.</p></div><h4>Primary Anthropogenic Cause: Fossil Fuel Combustion</h4><p>The burning of <strong>fossil fuels</strong> is the leading human-induced cause of acid rain. These fuels, particularly <strong>coal</strong> and <strong>oil</strong>, contain impurities like <strong>sulfur</strong> and <strong>nitrogen compounds</strong>.</p><p>When these fuels are combusted, they release significant amounts of <strong>sulfur dioxide (SO2)</strong> and <strong>nitrogen oxides (NOx)</strong> into the atmosphere. These are the primary precursor gases for acid rain.</p><div class='key-point-box'><p><strong>Key Pollutants:</strong> The two main gaseous pollutants responsible for acid rain are <strong>Sulfur Dioxide (SO2)</strong> and <strong>Nitrogen Oxides (NOx)</strong>.</p></div><h4>Sources of Sulfur Dioxide (SO2) Emissions</h4><p>A major contributor to <strong>SO2</strong> emissions is the combustion of <strong>coal</strong> in <strong>thermal power plants</strong>. Industrial processes, such as <strong>metal smelting</strong> and <strong>manufacturing facilities</strong>, also release substantial amounts of this gas.</p><div class='info-box'><p><strong>Major SO2 Sources:</strong></p><ul><li><strong>Coal-fired power plants</strong></li><li><strong>Industrial boilers</strong> and furnaces</li><li><strong>Smelters</strong> and other industrial processes</li></ul></div><h4>Sources of Nitrogen Oxide (NOx) Emissions</h4><p><strong>Nitrogen oxides (NOx)</strong> are primarily formed during high-temperature combustion processes. This includes emissions from <strong>motor vehicles</strong>, such as cars, trucks, and buses, due to the burning of petrol and diesel.</p><p>Additionally, <strong>industrial combustion processes</strong> and <strong>power generation</strong> also contribute to NOx emissions, especially when operating at very high temperatures.</p><div class='info-box'><p><strong>Major NOx Sources:</strong></p><ul><li><strong>Automobile exhaust</strong> (vehicles)</li><li><strong>Thermal power plants</strong></li><li><strong>Industrial furnaces</strong> and boilers</li></ul></div><h4>Natural Sources Contributing to Acid Rain Precursors</h4><p>While human activities are the dominant cause, natural phenomena also contribute to the presence of <strong>SO2</strong> and <strong>NOx</strong> in the atmosphere. These natural sources are generally less significant globally compared to anthropogenic ones.</p><ul><li><strong>Volcanic Eruptions:</strong> Volcanoes release large quantities of <strong>sulfur dioxide</strong> and hydrogen sulfide, which can be converted to SO2 in the atmosphere.</li><li><strong>Lightning:</strong> High-energy electrical discharges during lightning strikes cause atmospheric nitrogen and oxygen to react, forming various <strong>nitrogen oxides</strong>.</li></ul><h4>Atmospheric Chemical Reactions Leading to Acid Formation</h4><p>Once released into the atmosphere, <strong>SO2</strong> and <strong>NOx</strong> do not immediately form acid rain. They undergo a series of complex <strong>chemical reactions</strong> with water vapor, oxygen, and other chemicals present in the air.</p><p>These reactions transform the gaseous pollutants into droplets of <strong>sulfuric acid (H2SO4)</strong> and <strong>nitric acid (HNO3)</strong>. These acidic droplets then combine with water vapor in the clouds.</p><div class='key-point-box'><p><strong>Chemical Transformation:</strong></p><ul><li><strong>SO2</strong> oxidizes to form <strong>sulfuric acid (H2SO4)</strong>.</li><li><strong>NOx</strong> oxidizes and reacts with water to form <strong>nitric acid (HNO3)</strong>.</li></ul></div><p>When this acidic cloud moisture precipitates as rain, snow, or fog, it is termed <strong>acid rain</strong>. The acids can also deposit as dry particles, known as <strong>dry deposition</strong>.</p><div class='exam-tip-box'><p><strong>UPSC Insight:</strong> While natural sources exist, <strong>anthropogenic emissions</strong> from <strong>fossil fuel combustion</strong> are the primary focus for policy and mitigation strategies related to acid rain. Questions often revolve around these human-induced factors and their impacts.</p></div>
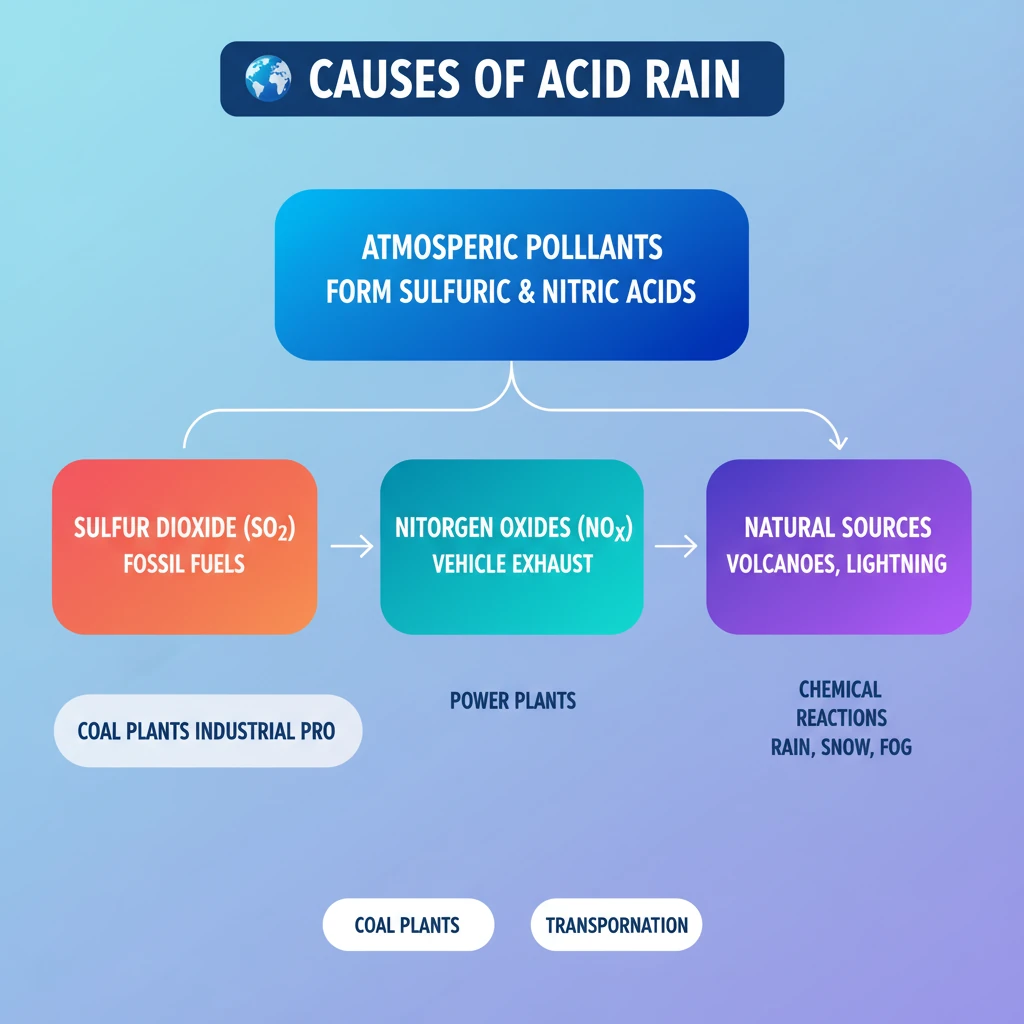
💡 Key Takeaways
- •Acid rain is precipitation with high levels of sulfuric and nitric acids, primarily caused by atmospheric pollutants.
- •The main anthropogenic causes are emissions of Sulfur Dioxide (SO2) and Nitrogen Oxides (NOx) from fossil fuel combustion.
- •Key sources include coal-fired power plants, industrial processes, and vehicle exhaust.
- •Natural sources like volcanic eruptions and lightning also contribute, but to a lesser extent.
- •SO2 and NOx undergo chemical reactions in the atmosphere to form sulfuric and nitric acids, which then fall as acid rain.
- •Acid rain has severe impacts on ecosystems, infrastructure, and human health, necessitating global and local mitigation efforts.
🧠 Memory Techniques
95% Verified Content
📚 Reference Sources
•United States Environmental Protection Agency (EPA) - Acid Rain Program
•National Geographic - Acid Rain Explained
•European Environment Agency (EEA) - Acidification
•NCERT Class 11/12 Environmental Chemistry (general knowledge)