What are the Key Findings of the Report? - Environment And Ecology | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search

What are the Key Findings of the Report?
Medium⏱️ 6 min read
environment and ecology
📖 Introduction
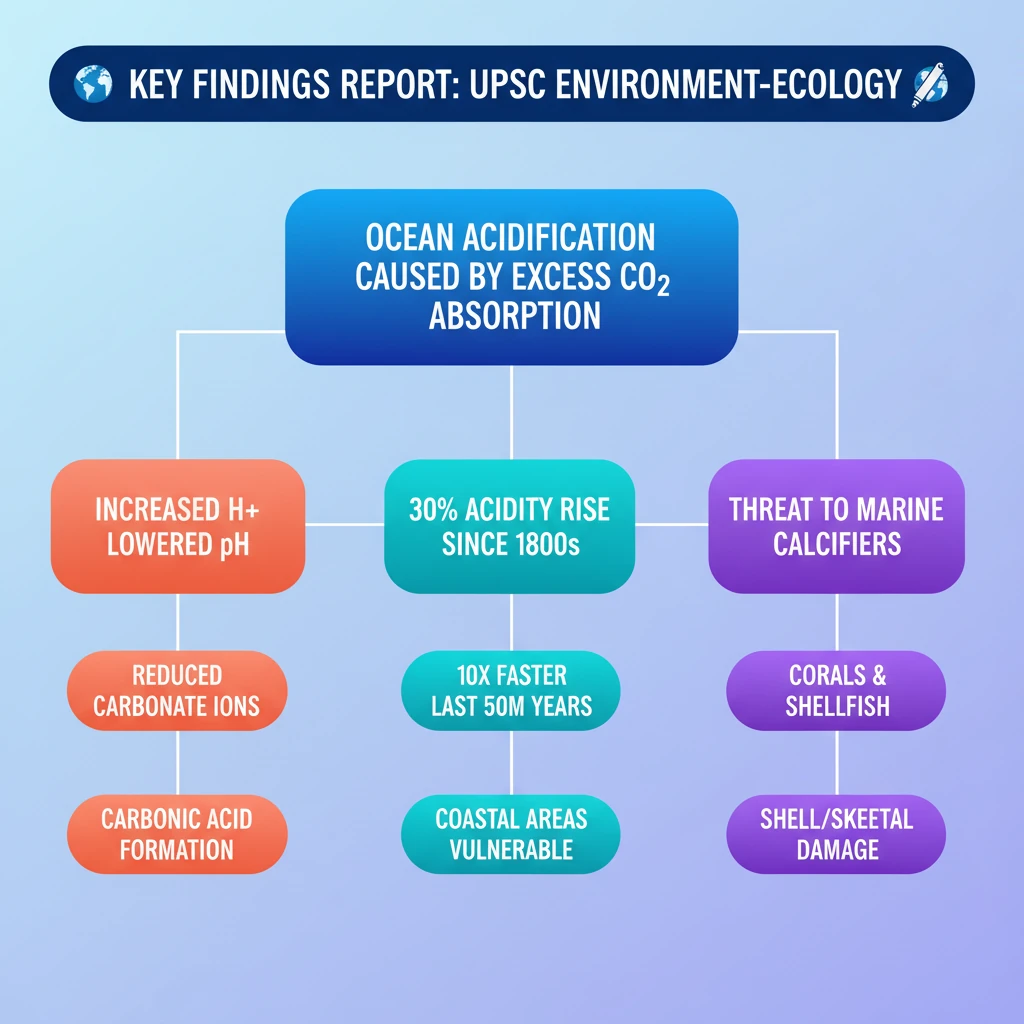
<h4>The Chemical Process of Ocean Acidification</h4><p>When <strong>carbon dioxide (CO2)</strong> is absorbed by seawater, it initiates a series of <strong>chemical reactions</strong>. These reactions lead to an increase in the concentration of <strong>hydrogen ions (H+)</strong> in the water.</p><p>Initially, <strong>CO2</strong> dissolves in water to form <strong>carbonic acid (H2CO3)</strong>. This acid is unstable and quickly dissociates into <strong>hydrogen ions (H+)</strong> and <strong>bicarbonate ions (HCO3−)</strong>.</p><div class='key-point-box'><p>The elevated presence of <strong>hydrogen ions (H+)</strong> directly contributes to an increase in the <strong>acidity</strong> of seawater. This process is known as <strong>ocean acidification</strong>.</p></div><p>A crucial consequence of this increased acidity is the reduction in the availability of <strong>carbonate ions</strong>. These ions are vital building blocks for many marine organisms.</p><h4>Accelerated Acidification due to Climate Change</h4><p>Oceans naturally play a critical role in absorbing <strong>atmospheric carbon dioxide</strong>. However, the unprecedented rise in anthropogenic <strong>CO2 emissions</strong> has led to oceans absorbing excessive amounts of this gas.</p><div class='info-box'><p>Since the <strong>1800s</strong>, the acidity of the oceans has increased by nearly <strong>30%</strong>. This rate of acidification is approximately <strong>10 times faster</strong> than any observed change over the last <strong>50 million years</strong>.</p></div><p>Projections indicate that if current emission trends persist, the <strong>surface ocean pH</strong> could significantly drop from its current average of <strong>8.1 to 7.7</strong> within the next <strong>100 years</strong>.</p><p><strong>Coastal areas</strong> face particular vulnerability. Factors like <strong>acid sulphate runoff</strong> from land and the exacerbating effects of <strong>climate change-related sea level rise</strong> intensify acidification impacts in these regions.</p><h4>Detrimental Impacts on Marine Organisms</h4><p>The fundamental change in seawater <strong>acidity</strong> poses severe threats to a wide array of <strong>marine organisms</strong>. Its effects are particularly detrimental to species that rely on <strong>calcium carbonate</strong> for their structural integrity.</p><ul><li><strong>Corals:</strong> Acidification hinders the ability of corals to form and maintain their <strong>calcium carbonate skeletons</strong>, leading to coral bleaching and degradation of entire reef ecosystems.</li><li><strong>Shellfish:</strong> Organisms such as oysters, clams, and mussels struggle to build and repair their protective <strong>calcium carbonate shells</strong> in more acidic waters, impacting their survival and reproduction.</li></ul><div class='exam-tip-box'><p>Understanding the specific impacts on <strong>keystone species</strong> like corals and shellfish is crucial for Mains answers on <strong>marine biodiversity</strong> and <strong>ecosystem health</strong> (<strong>GS Paper 3</strong>).</p></div>
💡 Key Takeaways
- •Ocean acidification is caused by oceans absorbing excess CO2, forming carbonic acid.
- •This increases hydrogen ions (H+), lowering ocean pH and reducing vital carbonate ions.
- •Acidity has increased 30% since the 1800s, 10 times faster than in the last 50 million years.
- •Coastal areas are highly vulnerable due to runoff and sea level rise.
- •It severely impacts marine organisms, especially those with calcium carbonate shells/skeletons like corals and shellfish.
🧠 Memory Techniques

95% Verified Content