What is the Haber-Bosch Process? - Agriculture Allied Sector | UPSC Learning
Topics
0 topics • 0 completed
🔍
No topics match your search
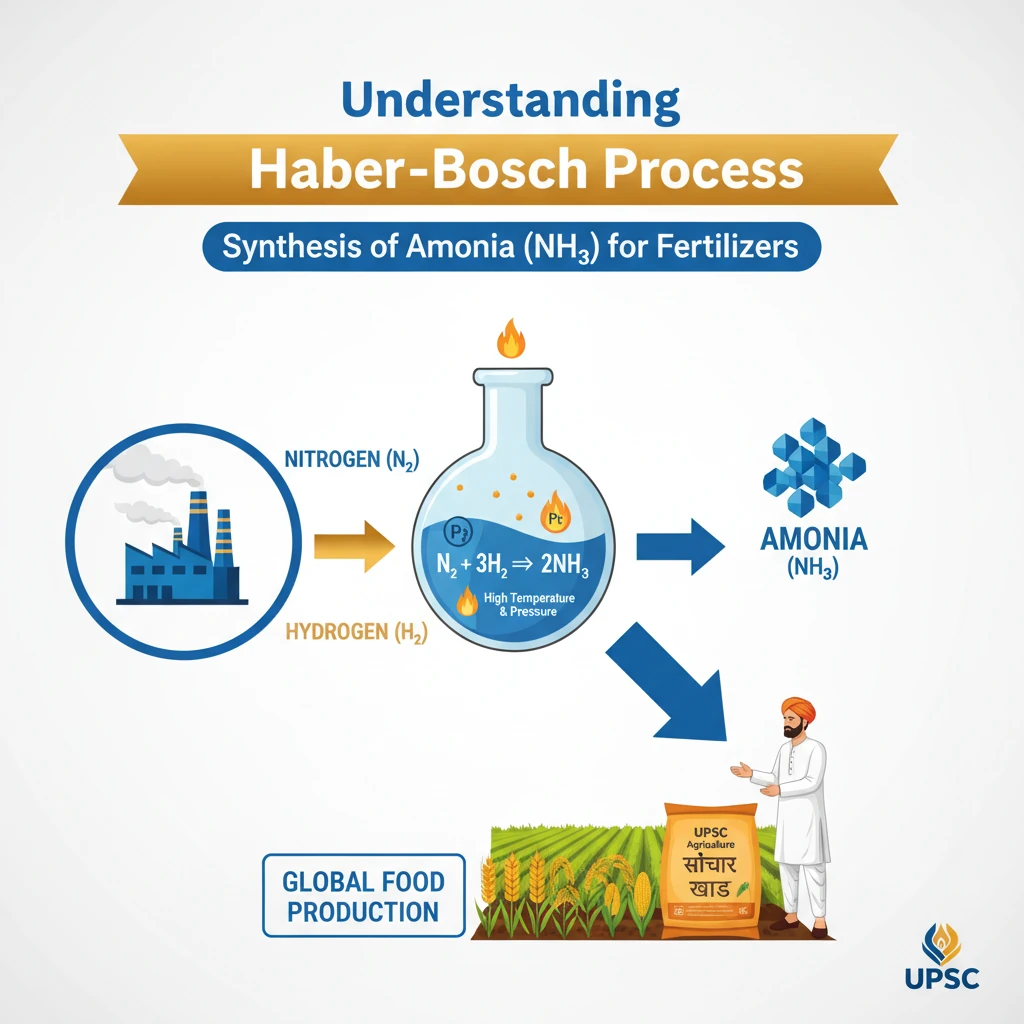
What is the Haber-Bosch Process?
Medium⏱️ 8 min read
agriculture allied sector
📖 Introduction
<h4>Understanding the Haber-Bosch Process</h4><p>The <strong>Haber-Bosch process</strong> is a crucial industrial method used for the synthesis of <strong>ammonia</strong> (NH3). This process combines <strong>nitrogen</strong> (N2) gas, primarily sourced from the air, with <strong>hydrogen</strong> (H2) gas.</p><p>Its primary significance lies in its substantial contribution to the global production of <strong>fertilizers</strong>. Without this process, large-scale food production would be significantly hampered.</p><div class="key-point-box"><strong>Key Concept:</strong> The Haber-Bosch process converts atmospheric nitrogen into a usable form (ammonia), making it accessible for agricultural and industrial applications.</div><h4>Industrial Setup and Conditions</h4><p>The reaction within the <strong>Haber-Bosch process</strong> occurs under very specific and demanding conditions to ensure efficiency and yield. A robust <strong>steel chamber</strong> is utilized to contain the reaction.</p><p>A high pressure of approximately <strong>200 atmospheres (atm)</strong> is maintained within this chamber. This elevated pressure is critical for effectively facilitating the chemical reaction between the <strong>nitrogen-hydrogen mixture</strong>.</p><div class="info-box"><strong>Process Parameters:</strong><ul><li><strong>Pressure:</strong> ~200 atm</li><li><strong>Temperature:</strong> Typically 400-450°C (not mentioned in source, but crucial for context)</li><li><strong>Catalyst:</strong> Iron-based catalyst (not mentioned in source, but crucial for context)</li></ul></div><p>A specially engineered <strong>valve</strong> is integrated into the system. This valve is designed to withstand the immense high pressure while simultaneously allowing the continuous flow of the <strong>N2-H2 mixture</strong> through the reaction chamber.</p><div class="exam-tip-box"><strong>UPSC Relevance:</strong> Understand the Haber-Bosch process not just as a chemical reaction, but as a cornerstone of modern agriculture and its implications for food security, energy consumption, and environmental impact (GS Paper III - Economy, Environment, Science & Technology).</div>
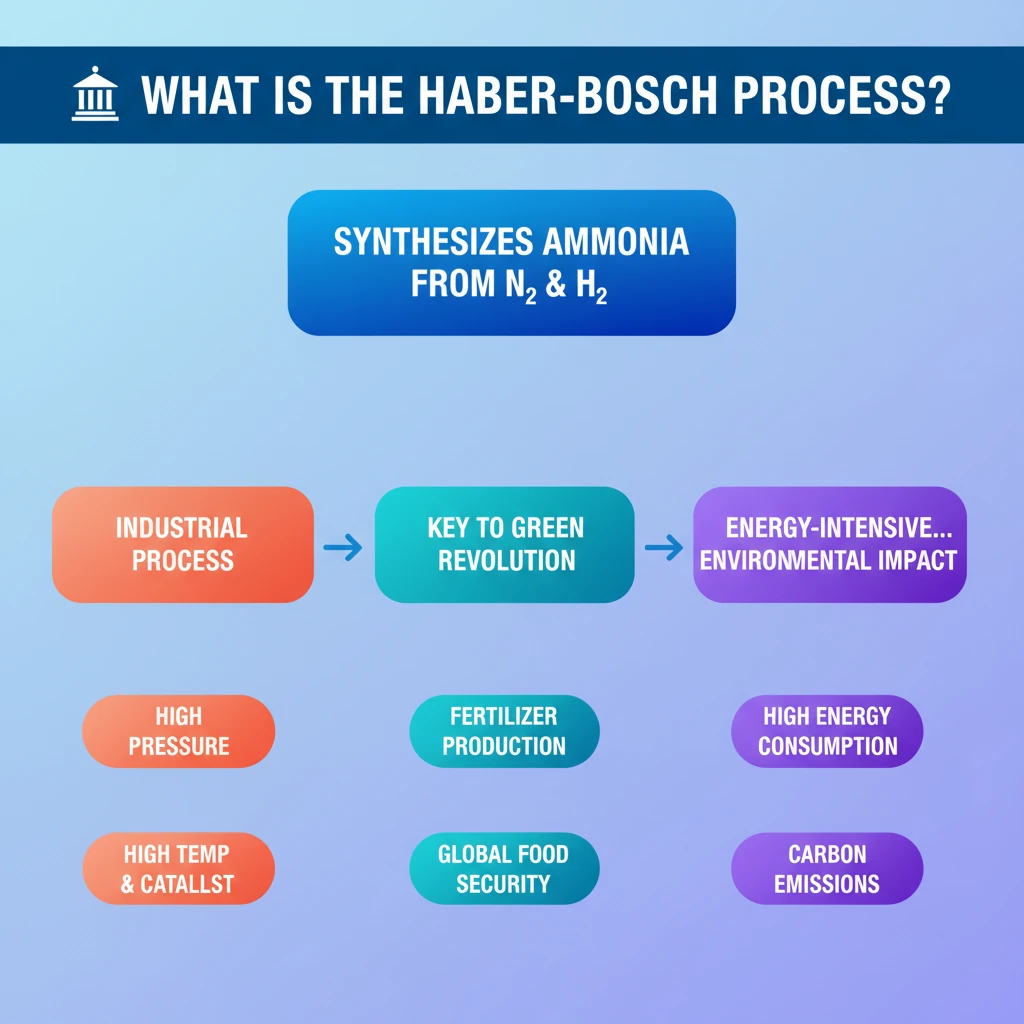
💡 Key Takeaways
- •The Haber-Bosch process synthesizes ammonia from atmospheric nitrogen and hydrogen.
- •It is an industrial process operating at high pressure (200 atm) and temperature with a catalyst.
- •Ammonia produced is primarily used for manufacturing nitrogen fertilizers.
- •This process was critical for the Green Revolution and global food security.
- •It is highly energy-intensive and has significant environmental implications.
- •Developed by Fritz Haber and industrialized by Carl Bosch in the early 20th century.
- •It remains indispensable for modern agriculture but faces challenges regarding sustainability.
🧠 Memory Techniques
98% Verified Content
📚 Reference Sources
•General Chemistry Textbooks (e.g., NCERT, Atkins' Physical Chemistry)
•Encyclopaedia Britannica (Haber-Bosch Process)
•NobelPrize.org (Fritz Haber, Carl Bosch biographies)